Review Article | DOI: https://doi.org/10.31579/2835-785X/036
Effect of COVID-19 Infection on Hemoglobin Level and Iron Metabolism
1 Department of Physiology, Faculty of Medicine, Sabratha University, Libya.
2 Department of Biomedical Sciences, School of Basic Sciences, Libyan Academy, Tripoli, Libya.
*Corresponding Author: Azab Elsayed Azab, Department of Physiology, Faculty of Medicine, Sabratha University, Libya.
Citation: Azab E Azab, J M Jbireal, and Maryouma N A Alnaas, (2024), Effect of COVID-19 Infection on Hemoglobin Level and Iron Metabolism International Journal of Clinical Research and Reports. 3(1); DOI:10.31579/2835-785X/036
Copyright: © 2024, Azab Elsayed Azab. This is an open-access artic le distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 18 January 2024 | Accepted: 14 February 2024 | Published: 26 February 2024
Keywords: iron metabolism; Hepcidin; COVID-19 infection; hemoglobin; Ferritin; iron dysregulation
Abstract
Background: The coronavirus disease 2019 (COVID-19) is an aggressive virus that spread worldwide and caused a pandemic infection. COVID-19 infection results in an inflammatory state involving a cytokine storm in COVID-19 patients. Interlukin-6 (IL-6) stimulates ferritin and the synthesis of hepcidin. Hepcidin sequesters iron in the enterocytes and macrophages, leading to increased intracellular ferritin, and preventing iron efflux from enterocytes and macrophages.
Objectives: The current review aimed to highlight the relationship between COVID-19 Infection, hemoglobin level, and iron metabolism among COVID-19 patients. On March 11, 2020, the World Health Organization declared COVID-19 a global pandemic. Therefore, the battle against COVID-19 is likely to be a marathon and the pandemic has a major impact on healthcare systems in many countries. Iron (Fe2+) metabolism is mainly regulated by the coordination between erythropoiesis and Fe stores. Wherever, ferritin level is increased, as it is an acute phase protein, it is always necessary to assess the underlying existence of inflammatory diseases, infectious diseases, and neoplasms. Pathologically, COVID-19 manifests itself in many complications as well as physiological and biochemical alterations. These include, but are not limited to acute respiratory distress syndrome, high concentrations of proinflammatory CD4 T cells and cytotoxic granules CD8 T, massive release of cytokines (cytokine storm), increased coagulation state, hemoglobin damage, and dysregulation of iron homeostasis including iron overload which is likely a major factor in the pathogenesis of COVID-19. Hyperferritinemia is largely considered an indicator of the hyperferritinemic syndromes (HFS) associated with severe COVID-19. Diagnosis of COVID-19 is related to the decreased hemoglobin, leukocytes-neutrophiles ratio, elevated D-dimer, and ferritin. There is a difference in the ability of COVID-19 proteins to form a conserved domain with porphyrin according to the number of amino acids and binding energy.
Conclusion: It can be concluded that COVID-19 manifests itself in many complications as well as physiological and biochemical alterations. The current review highlighted the relationship between COVID-19 infection, hemoglobin level, and iron metabolism among COVID-19 patients.
Introduction
The coronavirus disease 2019 (COVID-19) is an aggressive virus that spread worldwide and caused a pandemic infection. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a global pandemic. Therefore, the battle against COVID-19 is likely to be a marathon and the pandemic has a major impact on healthcare systems in many countries [1].
The biomarkers of iron metabolism are of interest in the novel Coronavirus Disease 2019 (COVID-19) because of the mounting evidence of the significance of iron status in immune responses [2]. Viral infections have the potential to cause hypoxia by directly affecting the respiratory system, which can set off an inflammatory cascade that results in anemia. Secondly, the bioavailability of iron could be reduced by activation of the innate immune system, which prevents the expansion of viral load in the acute-phase of the infection. This results in the activation of the iron-regulating peptide hormone hepcidin, which increases the retention of iron within enterocytes and macrophages—cells from which iron is typically mobilized predominantly for erythropoiesis. Hypoxia is the outcome of reduced erythropoiesis and elevated ferritin levels brought on by increased iron storage [2, 3]. The iron-storing protein ferritin is present in most cell types and is secreted into the serum by Kupffer cells, hepatocytes, macrophages, and maybe other cell types as well. Serum ferritin levels are assumed to represent body iron storage, making low levels a reliable sign of iron deficiency anemia [2, 4].
The study of Alnaas et al., [1] confirmed the observations variations in hematological parameters and some inflammatory factors in patients infected by covid-19. Authors recommended a further hematological studies are needed to confirm these results to help the clinicians for better understanding of COVID-19 infection and to provide more clinical treatment options.
Objectives
The current review aimed to highlight the relationship between COVID-19 Infection, hemoglobin levels, and iron metabolism among COVID-19 patients.
Iron metabolism and hepcidin
Iron (Fe2+) metabolism is mainly regulated by the coordination between erythropoiesis and Fe stores. Two mechanisms are mainly involved in the regulation of this homeostasis: the intracellular mechanism that is dependent on the cytoplasmic Fe store, and the systemic mechanism in which hepcidin plays a crucial role. In details, Dietary ferrous (Fe2+) is absorbed after binding to heme carrier protein 1 (HCP1) in the brush border membrane of the duodenal enterocytes and HCP1 imports it into the intracellular medium. Fe2+ is then released from the protoporphyrin by hemeoxygenase and can be stored as ferritin or exported to the blood [5, 6].
Importantly, Intracellular Fe2+ is exported to the plasma through ferroportin (FPT), a Fe exporting protein, and after the action of hephaestin, (Fe2+) is transformed into ferric (Fe3+) that binds to transferrin (Tf) and circulates in the plasma. There is no specific mechanism to eliminate the excessive iron resulting from the cellular uptake and recycling of red blood cells. Therefore, the homeostasis of serum Fe requires a coordination between the sites of absorption, utilization, and storage; this signaling is conducted by hepcidin [5].
Iron storage
Ferritin molecule is synthesized by the liver and exhibits the function of being an easily accessible intracellular Fe store. It is a molecule that comprises 24 heavy chain (21 kDa) and light chain (19 kDa) subunits [7]. The synthesis of ferritin subunits is regulated by ribonucleic acid (RNA) transcription in the hepatocytes, which is induced after the binding of iron regulatory proteins (IRP) to an iron responsive element (IRE), (Figure. 1). When intracellular Fe concentration is low, the binding of IRP to IRE suppresses the response for the production of ferritin. Conversely, when the intracellular Fe concentration is high, IRP is degraded, making its binding to the IRE impossible and then leading to ferritin synthesis [8].
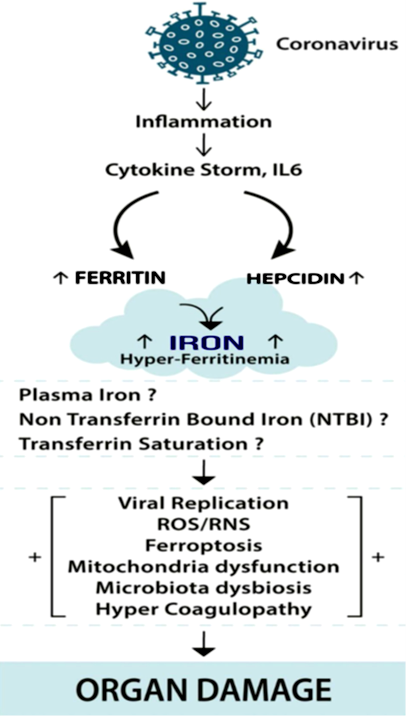
Figure 1: COVID-19 infection and Iron dysregulation. COVID-19 infection results in an inflammatory state involving a cytokine storm in COVID-19 patients. Interlukin-6 (IL-6) stimulates ferritin and the synthesis of hepcidin. Hepcidin sequesters iron in the enterocytes and macrophages, leading to increased intracellular ferritin, and preventing iron efflux from enterocytes and macrophages. Excess intracellular iron interacts with molecular oxygen, generating reactive oxygen species (ROS) through Haber-Weiss and Fenton reactions and reactive nitrogen species (RNS) and reactive sulfur species (RSS). The intracellular iron excess leads to ferroptosis, a process of programmed cell death. Iron overload may also affect extra and intracellular mitochondria function and microbiota diversity (lungs and gut) and blood coagulation [9].
Serum ferritin (SF) concentration is a reliable marker of the body Fe reserves [10]. In situations where ferritin level is increased, as it is an acute phase protein, it is always necessary to assess the underlying existence of inflammatory diseases, infectious diseases, and neoplasms. It is necessary to assess whether there is an iron overload (IO) that can be determined by high transferrin saturation (TS) [11].
Pathologically, COVID-19 manifests itself in many complications as well as physiological and biochemical alterations. These include, but are not limited to acute respiratory distress syndrome (ARDS) [12], high concentrations of proinflammatory CD4 T cells and cytotoxic granules CD8 T [13], massive release of cytokines (cytokine storm) [14], increased coagulation state [15], hemoglobin damage [16] and dysregulation of iron homeostasis [17] including iron overload [18, 19] which is likely a major factor in the pathogenesis of COVID-19.
Variation in Hemoglobin concentration, Serum iron, Ferritin level, and Iron overload due to COVID-19 infection
Haemoglobin
Because of its heme ring and the iron in its structure, hemoglobin (Hb) is able to transfer oxygen from the lungs to various tissues [20]. Therefore, a disorder in the iron metabolism and hemoglobinopathy may remarkably compromise the capacity of red blood cells to transport O2 [21]. Conversely, haemoglobin disorders are generally not associated with respiratory conditions. However, complications involving the heart, lungs and the immune system, can be present in these patients and in a SARS-CoV-2 positive patient may trigger very serious complications.
Additionally, Diagnosis of COVID-19 is related to the decreased Hemoglobin, leukocytes-neutrophiles ratio, elevated D-dimer, and ferritin. The violent immune response of the human body against the COVID-19 virus causes the release of ''cytokines storm'', resulting in ROS production's cascade leading to hypoxia [22, 23]. Moreover, the hypothesis assumed that the generation of excess reactive oxygen species leads to hypoxemia, further cell stress, and heme [24] destruction. Oxygen supplementation fails in critical cases due to the severe hypoxia caused by the virus. The prognosis and pathophysiology of COVID-19 are not the same as the rest of the Coronavirus family and is still poorly understood [25].
Various hypotheses about the relation between the COVID-19 and the 1-beta Hemoglobin chain were assault. Theoretical research relies on the ability of COVID-19 proteins with porphyrin to form a conserved domain and release free iron leading to a drop in the affinity of Hemoglobin binding to oxygen and interfere with the Hemoglobin anabolism [26]. In more details, there is a difference in the ability of COVID19 proteins to form a conserved domain with porphyrin according to the number of amino acids and binding energy (Table 1).
Table 1: Conserved domains between structural proteins and nonstructural proteins of COVID-19 with porphyrin according to the number of amino acids bound to porphyrin and binding energy [27].

Theories of COVID-19 attack on Hemoglobin.
The first theory according to the study of Liu and Li [25] COVID19 Hemoglobin (Hb) metabolism will interfere with the formed conserved domain and separate harmful irons in the blood, which leads to a significant increase in serum ferritin, albumin, erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), and C-reactive protein (CRP), and decrease neutrophils whereas the second theory found that there is no evidence between COVID-19 and Hemoglobin defect7 experimentally [28]. Additionally, they found no Hb affinity variation to carry oxygen in positive COVID-19 patients, so no Hb value variation means no association between Hb and COVID-19 [28, 29]. Patients with COVID-19 have a higher incidence rate of Thromboembolism [30] and no relation between it and Hb toxicity. No absorbance occurs to free heme when measured at the wavelength range [31].
As a result of those studies, a link between COVID-19 infection symptoms and the consequences of excess heme does not have to be relevant for every patient; but, in certain circumstances, it may correlate or even create a more severe illness development due to pre-existing hemolytic diseases or hemolysis-provoking events [31].
Role of non-beta hemoglobin in Covid-19
On the other hand, with regard to the role of Fetal Hemoglobin in COVID-19 the studies had revealed that Hemoglobin F (HBF) may be one of the main reasons for decreasing the prevalence of COVID-19 in pediatrics [32] The hypothesis based on the molecular docking study approved that SAR-COV2 attacks the Beta chain, causing a release of iron from porphyrin and iron toxicity [26] The pilot study results showed that patients with hemoglobinopathies like beta-thalassemia and sickle cell anemia had low mortality and fatality rates toward COVID-19 [32]. Therefore, From the previous results of studies, some clinical trials started to use HBF inducers agent s in protocols for treatment COVID-19 like Hydroxyurea and Panobinostat [33].
There were many studies have demonstrated the implication of the Covid-19 infection in hemoglobin decrease, high levels of ferritin and relatively iron overload. Accordingly, it has previously been demonstrated that SARS-CoV-2 (Covid-19) binds to hemoglobin through ACE2, CD147, Cd26, and other receptors that are present on the surface of erythrocytes. After this association, virus attacks the beta1 chain of hemoglobin which leads to dysfunctional hemoglobin in addition to hemolysis, thereby reducing the oxygen supply to the body, causing tissue hypoxia, a remarkable characteristic of COVID-19 [21, 34].
One of the important studies has conducted that the amino acid sequence of the coronavirus spike protein is identical to hepcidin, a protein that acts as the main systemic regulator of iron metabolism. Therefore, this similarity between hepcidin and coronavirus spike protein can lead to a mimetic effect, suggesting that SARS-CoV-2 can increase serum hepcidin and then ferritin, and cause hyperferritinemic syndrome [35]. Taking into consideration that viruses generally stimulate increased iron deposition in the host cells, laboratory findings commonly found in patients with COVID-19, such as low hemoglobin, hyperferritinemia, low serum iron, thrombocytopenia, increased red cell distribution width (RDW) and (LDH), suggest that the hypothesis of the dysregulation of iron metabolism associated with inefficient erythropoiesis is a possible mechanism underlying the pathophysiological changes in patients with COVID-19. On the other hand, Viral infection is well known to be associated with abnormal haematological parameters. Autopsy of patients who died of COVID-19 showed markedly shrunken spleen with reduced lymphocyte, macrophage proliferation, and phagocytosis [36]. Lymphocytes were also depleted in lymph nodes, and all haematopoietic cell lineages were reduced in the bone marrow.
In another study has been done in Libya where thirty confirmed COVID-19 patients hospitalized in the Isolation Centre located in Sabratha city, Libya from the 2nd October 2020 to the 15th March 2021. They found that patients with COVID-19 had a significant (P=0.0088) decrease in hemoglobin concentration [(median (IQR) g/ dl], 13.35 (11.73-14.00), 13.05 (12.10-14.05), and 12.60 (11.45-13.60) at 0 day, 14 days, and 21 days of infection, respectively compared with the healthy individuals (13.95 (12.70-15.53) [37].
Importantly, the experimental research argues the link between COVID-19 and Hemoglobin clinical laboratory results from 21 patients positive to COVID-19 to 21 patients with ARDS but without COVID-19 [28]. Another study in elderly patients hospitalized for COVID-19 found that most patients had hemoglobin levels lower than the normal range, but did not find significant differences in hemoglobin levels between survivors and non-survivors [38]. Similarly, in a report of 5700 patients hospitalized for COVID-19 in the New York City area, ferritin levels were pathologically high [39].
For hemoglobin concentration, based on findings from 139 observational studies comprising 40,450 individuals, pooled mean hemoglobin level was 129.7 g/L (95% Confidence Interval (CI) 128.51; 130.88; I 2=98.2%, P-value for heterogeneity ˂ 0.001). Compared to moderate COVID-19 cases (using data from 63 studies with 21,605 individuals), severe cases had lower hemoglobin levels [weighted mean difference (WMD), −4.08 g/L (95% CI −5.12; −3.05); I2 =59%, P-value for heterogeneity ˂ 0.001 [40].
Also, Huang et al. [41] reported reduction in hemoglobin levels in 38.2% of hospitalized COVID-19 patients, but did not specify the definition of decreased hemoglobin. While Wang et al. [42] reported reduced hemoglobin levels (˂ 110 g/dl) in 19.23% of the study population admitted to hospital.
Serum iron
Iron is an essential element for all living cells as it is key to establishing many functioning metabolic processes including dioxyribonuclic acid (DNA) synthesis, DNA repair, DNA transcription, energy production, oxygen transport, oxygen storage, and drug detoxification [43]. Interaction of Covid-19 with iron metabolism and low oxygen supply may be related to ancestral phylogenetic mechanisms, which date back to environments with low oxygen and highly available Fe levels. Alternatively, the evolution of viral replication is well adapted to this type of microenvironment, where the Fenton oxidative reaction is favored [44, 45].
Pathologically, non-transferrin or non-ferritin bound iron, also called catalytic iron, leads to the generation of reactive oxygen species such as hydroxyl radicals through the Fenton reaction [46]. High catalytic iron levels in plasma are associated with mortality and adverse clinical events in patients with a variety of acute illnesses, including acute coronary syndromes, cardiogenic shock, and multi-organ failure with acute kidney injury (AKI) [47, 48].
Extremely important, recent studies implicated that ferroptosis, which is the process of programmed cell death mediated by iron dependent peroxidation mechanisms [49] in inflammatory pathologies, involves multiple organs including liver, kidney, heart and lung. Significantly, in another study where 127 patients have been diagnosed with COVID-19 at Centro Hospitalar e Universitário de São João (CHUSJ), Porto, Portugal, between 2 September, and 17 November 2020 [50]. COVID-19 diagnosis was based on SARS-CoV-2 RNA detection by reverse-transcription real-time polymerase chain reaction (PCR) in a nasopharyngeal swab sample. This study has revealed that Both COVID-19-positive and COVID-19-negative patients had lower serum iron at Hospital admission than healthy blood donors. Moreover, COVID-19- positive patients had significantly lower serum iron levels than COVID-19-negative patients (25 (17–42) versus 42 (23.3–76.8) µg/dL). Defining hypoferremia as serum iron level lower than 50 µg/dL, 80.7% of COVID-19-positive and 57.1% (p < 0>
Iron overload
Generally, the importance of intracellular iron for viral replication is well known, and as reported by Armitage in 2014 [51]. There is a close relation between hepcidin and iron regulation in HIV-1, HBV, and HCV patients. This relationship has been described for hepatitis virus C already in the 2000s by treating patients with the use of phlebotomy to promote iron reduction to improve interferon treatment response [52].
Recently, Smidth [45] underlines the importance of intracellular iron for viral replication, and this replication is influenced via Human Hemochromatosis Protein (HFE) and hepcidin. The basic consequence of this mechanism is the concentration of intracellular iron and the reduction of the extracellular one. As we have mentioned above, hepcidin is one of the most important regulators in iron balance. It has two main functions: the first one is to store iron into the cell to allow cellular duplication an DNA and RNA synthesis; the second one is its antibacterial effect by depriving the microorganism of iron for replication outside the host. This mechanism prevents bacterial infection but could facilitate viral replication. Its effect acts on duodenalenterocytes and the reticuloendothelial system by promoting ferroportin degradation which reduces enteric absorption of iron but retaining iron in the cell [53, 54].
Interestingly, the excess of this intracellular iron is excreted as ferritin. As reported by Clark and Pedz-ernik [55], intracellular iron surplus results in the detachment of Iron-Regulatory protein (IRP) from the Ferritin mRNA, with consequent ferritin formation. Importantly, Connelly, in 1997 [56], related high serum ferritin levels as a predictor of ARDS, which is one of the main problems in the actual COVID infection. How ARDS or acute lung injury (ALI) occurs was first described by Dixon in 2012 [57] as “Ferroptosis,” and recently, Liu et al, [58] investigated this in COVID infection, proposing iron chelation as a beneficial adjuvant in treating these patients.
Moreover, Huang [59] has reported an excessive redox-active iron mobilization in lung injury induced by ischemia, from intracellular iron accumulation to the vascular space, and iron stress in the vascular space could enhance the generation of highly damaging reactive oxygen species extracellularly. These data suggest that COVID infection has a major effect on iron by the hepcidin pathway and this mechanism is related to iron overload and hepcidin, by increasing intracellular iron, promoting pulmonary ferroptosis, mobilizing the iron in vascular space, and activating coagulation via an independent pathway. Clinically, In the literature, we can find a various number of substances that interact with hepcidin, inducing, or inhibiting it [60-63] in different ways and with different intensity. Among inhibitors we found: enoxaparin, fondaparinux, momelotinib, imatinib, spironolactone, siltuximab, tocilizumab, curcumin, dorsomorphin (small molecule), aspirin, Angelica Sinensis polysaccharide, and many others (Figure.2).
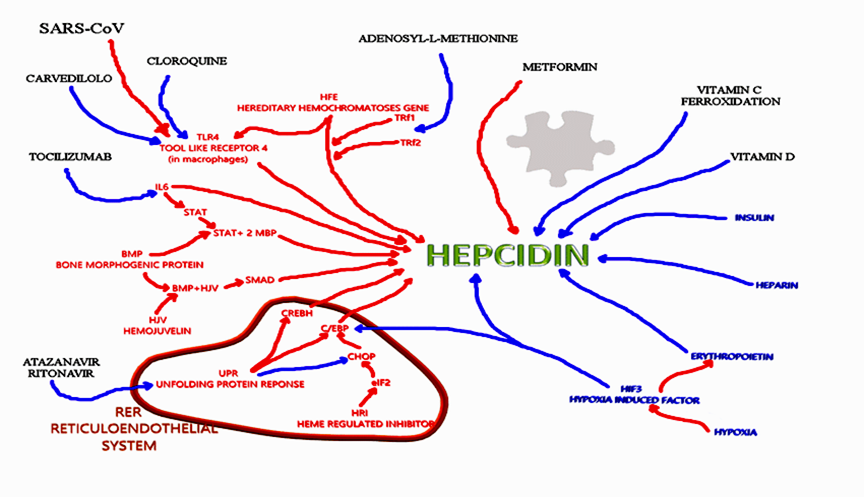
Figure 2: Hepcidin interaction. Molecules that have interactions with hepcidin. Red words: endogenous molecules stimulating hepcidin; Blue words: endogenous molecules inhibiting hepcidin; Black words: exogenous molecules effecting on Hepcidin; Red arrow: way of stimulation; Blue arrows: way of inhibition [64].
Ferritin level
Hyperferritinemia is largely considered an indicator of the hyperferritinemic syndromes (HFS) associated with severe COVID-19. This condition characterizes several autoimmune diseases [65] and due to its immunomodulatory properties may play a pathogenic role [9]. Currently, it is well known that many COVID-19 patients with a raised serum ferritin level of >300 μg/l had a 9-fold increase in the chances of death before discharge [66, 67].
Intrinsically, ferritin is the primary site of iron storage in the cell mainly in its ferric state (Fe3+). Ferritin can carry up to 4500 iron molecules in its core [68]. Generally, systemic inflammations are associated with increased serum ferritin levels. During a heightened inflammatory state, cytokines, particularly IL-6, stimulate ferritin and hepcidin synthesis [69]. Although circulating serum ferritin’s source during inflammatory conditions is still uncertain, in vitro experiments provide the possibility of it being secreted through hepatocytes [70] and by macrophages through a nonconventional pathway [71]. This demonstrates the likelihood of a contribution to ferritin production through macrophage activation in hyperferritinemic syndromes. Generally, ferritin is considered as a marker of iron stores, but also long known as an acute phase reactant due to either transcriptional or post-transcriptional regulation by pro-inflammatory cytokines [72]. In clinical practice, ferritin has been also frequently included in routine evaluation of COVID-19 at hospital admission [73]. Additionally, ferritin has been included in clinically used inflammatory marker panels, but little is known about its cellular uptake or release and its downstream fate after endocytosis [74]. Moreover, physiologically there are 3 main regulatory pathways for the expression of ferritin [74] driven by increased serum iron, hypoxia, and inflammation. During inflammation, ferritin may be released from macrophages or cells owing to tissue damage and may be regulated by tumor necrosis factor α, interleukin 2, and interleukin 10.
It is important to note that the concomitant presence of high serum ferritin levels and low iron levels can be observed during COVID-19 [75]. Furthermore, despite some inconsistency, many retrospective studies and a smaller number of prospective studies have shown that either the state of hyperferritinemia or hypoferremia are accompanied by a greater severity/worse prognosis of COVID-19 both in its acute and post-acute phase.
It is extremely important to mention here, that serum ferritin concentrations have been shown to reflect the status of iron stores in healthy individuals with low ferritin concentrations indicating iron deficiency, while elevated concentrations point to iron overload [76].
Experimentally, Mehta et al. have reported an increase in serum ferritin levels in patients with severe clinical severity of COVID-19 and serum ferritin levels can also be used as a predictor of the severity of COVID-19 disease and are associated with the presence of cytokine storms in these patients, in line with research conducted by at Jinyintan Hospital Wuhan China in December 2019, increased ferritin was associated with death in COVID-19 patients (P ˂ 0. 001) also reported that, as patients recovered, ferritin and interleukin-6 (IL-6) concentrations decreased. This can confirm that hyperferritinemia is associated with the inflammatory state in SARS-CoV-2 infection [77- 79].
Additionally, although ferritin is reported as an acute-phase protein, there is literature lacking in reporting the particular modified levels, leading to a misunderstanding regarding its interpretation [80]. In a recent meta-analysis published by Zeng [81], ferritin had been considered only in 4 of the 16 studies analyzed, but he underlined that ferritin levels could classify COVID patients’ severity. This reflects the fact that very few studies at the moment consider iron metabolism in COVID and non-COVID patients.
Physiologically, ferritin production occurs when intracellular iron concentration augments, with iron being stored in the form of ferritin and subsequently expelled from the cell. Intracellular iron accumulation occurs in two main modalities: hyperexpression of Transferrin receptor 1 which internalizes transferrin, and hepcidin expression. Hepcidin inhibits iron ions expulsion blocking ferroportin, which is the only siderophore of the cells [82].
Collectively, low iron levels can lead to anemia, whereas high levels cause excessive oxidative stress that damages cells/organs. On the other hand, both low and high body iron levels increase the risk of infection [83]. Those aspects show the significance of iron homeostasis in the body. As such, independent of COVID-19, a pattern of high serum ferritin (iron-storage protein) but low serum iron and low transferrin (iron-carrier protein in circulation) within 3 days of intensive care unit (ICU) admission has been seen in more than 75% of critically ill patients [83], indicating the significance of iron and related proteins in critical illness, and critical illness is seen in COVID-19 cases [84].
Clinically, in a meta-analysis of 25 retrospective observational studies (n = 5350) [85], it was shown that C-reactive protein (CRP), pro-calcitonin (PCT), d-dimer, and ferritin are associated with a worsening picture that includes mortality, severe COVID-19, ARDS, and need for ICU care in patients with COVID- 19. Additionally, ferritin seems to have more than a role as an indicator or consequence of this inflammation, but also actively participates in this process. Ruddell et al. demonstrated in rat hepatic stellate cells (HSC) that ferritin, both H-chain rich (FTH) and L-chain rich (FTL), is more than a consequence of inflammation, but also a mediator of it, through a pathway independent of iron and the TIM-2 receptor [86].
Furthermore, Zhang et al. [87] performed laboratory tests in patients with different severity levels of COVID-19 (mild, severe and extreme severe) and also reported the significant increase of ferritin in patients as the severity of disease (p˂ 0.01), especially when comparing the mild group with the extreme severe group. In addition, CD4+ T cells, CD8+ T cells and B cells gradually decreased with disease severity, showing to be negatively correlated.
The effect of covid-19 infection on hemoglobin, serum iron and serum ferritin levels
Review of the most important literatures published recently about the effect of covid-19 infection on hemoglobin, serum iron and serum ferritin level are listed in the following tables (2-4).
Table 2: The main findings of hemoglobin concentration in (covid-19) patients.
References | Region | Study Period | Sample Size | Mild patient | Severe patient | Main Finding |
| kantri et al., [88] | Morocco | March 18, 2020 until May 20, 2020 | 134 | 13,2g/l | 12.2g/l | Anemia was not common in this study. |
| Lino et al., [89]. | Brazil | May through July 2020 | 97 | 10.6 ± 2.2 g/dL | 9.9 ± 2.8 g/dL | Decrease in hemoglobin level. |
| Anai. et al., [90] | Japan | 2020 | 23 | 13.5 g/dL | 11.5 g/dL | Showed the decrease In hemoglobin. |
| Urrechaga et al., [91] | Spain | March and April 2020. | 1,336 | 13.2 g/dL | 12.6 g/dL | decreased hemoglobin in 40-50 % of cases. |
| Tao et al., [92] | Wuhan, China | 1 December 2019 to 20 March 2020 | 375 | 12.8 g/dL | 11..5 g/dL | anemia was an independent risk factor associated with severe illness in COVID‐19. |
| Yağc et al., [93] | İstanbul, Sisli | March and June 2020 | 59 | 14.5 g/dL | 13.5 g/dL. | Hemoglobin (Hb) levels were significantly lower in the critical patient group (P < .0001) and deceased group (P < .0001). |
Table 3: The main findings in some reviewed studies with regard to ferritin level.
References | Region | Study Period | Sample Size | Miled patient | Sever patient | Main finding |
| Tural-Onur et al., [94] | Istanbul, Turkey | Between 11 March–20 April 2020 | 301ng/ml | 451 ng/ml | 1145.7 ng/ml | shown that ferritin which is an indicator of systemic inflammation can be a predictor of disease severity and mortality. Ferritin values are higher in the non-survivor group despite treatment (p < .01). |
Lino et al., [89]
| Brazil | May through July 2020, | 97 | 1717.7 ± 2789.8ng/ml | 4207.7 ± 3530.3ng/ml | Early hyper inflammation in COVID-19 patients evaluated the high levels of serum ferritin in the first seven days of hospitalization. |
| Chen et al., [95] | in Wuhan, China | Jan 1 to Jan 20, 2020. | 99 | 490.7 ng/ml | 808.7 ng/ml | 63 of patients had serum ferritin way above of the normal range. |
| Banchini. et al., [82] | Italy | February 27 to April 28, 2020 | 101 | 761.2 ng/ml | 1198.6 ng/ml | ferritin was increased |
Table 4: The main findings in some reviewed studies with regard to serum iron measurement
References | Region | Study Period | Sample Size | Milled patient | Sever patients | Main finding |
| Zhao et al., [96] | Hospital of Wuhan University | February 1 to February 29, 2020. | 50 | 6.6μmol/L | 4.9μmol/L | (90%) patients had abnormally low serum iron levels (<7> |
| Sonnweber et al., [97] | Austria | 2020 | 102 | 18 ± 6(μg/L) | 15 ± 6(μg/L) | 30% of patients still presented with iron deficiency |
| Bolondi et al., [98] | Hospital in Cesena (Italy). | from the 6th of March to the 6th of April 2020 | 31 | 58(μg/L) | 45(μg/L) | acute respiratory distress syndrome (ARDS) causes reduced serum iron levels |
| Alipour et al., [99] | Iran | April- 2020 | 18 | 69(μg/L) | 27(μg/L) | serum iron levels were lower than normal range in admitted patients and serum Iron levels in ICU admitted patients were significantly lower than the others. |
Conclusion
It can be concluded that COVID-19 manifests itself in many complications as well as physiological and biochemical alterations. The current review highlighted the relationship between COVID-19 infection, hemoglobin level, and iron metabolism among COVID-19 patients.
References
- References
View at Publisher | View at Google Scholar

 Clinic
Clinic
