Research Article | DOI: https://doi.org/10.31579/2834-8427/013
Extant interspecific hybridization among trematodes within the Schistosoma haematobium species complex in Nigeria
1Department of Animal and Environmental Biology, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria.
2Disease Intervention and Prevention Program, Biomedical Research Institute, San Antonio, TX, USA.
3Department of Biological Sciences Federal University, Dutse, Jigawa state, Nigeria.
4Department of Biosciences and Biotechnology, University of Medical Sciences, Ondo, Ondo state, Nigeria.
5Molecular Parasitology Section, Laboratory of Parasitic Diseases, NIAID, National Institutes of Health, Bethesda.
*Corresponding Author: Ajakaye OG , Environmental Biotechnology and Sustainability Research Group, Department of Plant Biology and Biotechnology, University of Benin, Nigeria. And Molecular Parasitology Section, Laboratory of Parasitic Diseases, NIAID, National Institutes of He
Citation: Ajakaye OG , Enabulele EE , Balogun JB, Oyeyemi OT , Grigg ME, (2023). Ex-situ mycoremediation of petroleum polluted soils in Ogoniland, Nigeria, J Clinical Gynaecology and Breast, 2(3); DOI: 10.31579/2834-8427/013
Copyright: © 2023, Ajakaye OG. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 31 March 2023 | Accepted: 14 June 2023 | Published: 26 June 2023
Keywords: schistosoma haematobium; hybrids; genetic diversity; nigeria
Abstract
Background: Natural interspecific hybridization between the human parasite (Schistosoma haematobium [Sh]) and bovine par- asites (S. bovis [Sb], S. curassoni [Sc]) is increasingly reported in Africa. We developed a multi-locus PCR DNA-Seq strategy that amplifies two unlinked nuclear (transITS, BF) and two linked organellar genome markers (CO1, NAD5) to genotype S. haematobium eggs collected from infected people in Ile Oluji/Oke Igbo, Ondo State (an agrarian community) and Kachi, Jigawa State (a pastoral community) in Southwestern and Northern Nigeria, respectively.
Principal Findings: We applied this methodology against 57 isolates collected from a total of 219 participants. All patients from Jigawa state were infected with just one of two haplotypes of an S. haematobium x S. bovis hybrid based on sequences obtained at CO1, NAD5, transITS and BF markers. Whereas samples collected from Ondo state were varied. Mito- nuclear discordance was observed in all 17 patients, worms possessed an Sb mitochondrial genome but one of four different haplotypes at the nuclear markers, either admixed (heterozygous between Sh x Sc or Sh x Sb) a both markers (n=10), Sh at BF and admixed at transITS (Sh x Sc) (n=5), admixed (Sh x Sc) at BF and homozygous Sc at transITS (n=1) or homozygous Sh at BF and homozygous Sc at transITS (n=1).
Significance: Previous work suggested that zoonotic transmission of S. bovis in pastoral communities, where humans and ani- mals share a common water source, is a driving factor facilitating interspecific hybridization. However, our data showed that all isolates were hybrids, with greater diversity identified in Southwestern Nigeria, a non-pastoral site. Further, one patient possessed an S. bovis mitochondrial genome but was homozygous for S. haematobium at BF and homozygous for S. curassoni at transITS supporting at least two separate backcrosses in its origin, sug-gesting that interspecific hybridization may be an ongoing process.
Author Summary: Interspecific hybridization between trematode parasites poses serious health risks to humans. Many systems have shown possible hybridization between different schistosome species. As evidence of natural hybridization be- tween human S. haematobium and animal S. bovis or S. curassoni has grown in recent years, epidemiological surveys across potential hybrid zones are required, particularly in endemic African regions. According to severalreports, indiscriminate human-animal water contact is a major factor contributing to hybridization of human and animal schistosomes. We collected and genotyped 57 parasite isolates from pastoral and non-pastoral communi- ties in Kachi, Jigawa state, and Ile Oluji/Oke Igbo, Ondo state, Nigeria to screen for hybrids. In both sites, we foundSchistosoma hybrids with mitonuclear discordance and repeated backcrossing between S. haematobium, S. bovis,and S. curassoni. Contrary to previous reports, Schistosoma hybrids appear to be widespread and not solely de pendent on human-animal water interactions.
Introduction
Schistosomiasis is a highly prevalent water transmitted disease classified by the World Health Organization (WHO) as neglected. It is the second most prevalent tropical disease caused by five major species of flat worms, specifically Schistosoma mansoni, S. haematobium, S. japonicum, S. mekongi, and S. intercalatum. The first two species cause approximately 20 million infections in Africa and are associated with severe chronic health conse- quences in affected populations [1&2]. Many other schistosome species, including S. bovis, S. curassoni, S. mat- theei are known to infect livestock especially in sub–Saharan Africa [3]. Nigeria has the highest number of schis- tosomiasis cases in the world, with over 26% of Nigerians requiring chemotherapy. Despite annual mass chemo- therapy programs, the urogenital form of schistosomiasis, caused by S. haematobium, remains a significant public health problem, with a current national prevalence of about 10% [4]. In 2009, field evidence of hybridization between S. haematobium and the bovine schistosome S. bovis was reported for the first time in Africa based on typing strategies using the mitochondrial marker Cox1 (CO1) and the transITS (ITS) nuclear marker, located within the ribosomal RNA gene array [5]. Since the 2009 report, hybrid S. haematobium infections are now widely reported in countries in Africa, including Nigeria [6, 7,]. Whether such interspecific hybridization impacts urogenital schistosomiasis transmission, virulence, and treatment drug efficacy remains enigmatic More recently, several genome sequencing projects investigated S. haematobium [8-10] and S. bovis [11] at whole genome resolution to infer genome ancestry. Hybrid ancestry was extant, but the proportion of the nuclear genome derived from S. bovis was limited, resulting in only 8.2% [8] or 23% [10] within the S. haematobium ge- nomes investigated. The failure to identify full genome hybrids was interpreted to suggest that interspecies hy- bridization between S. haematobium x S. bovis is ancient, that it has occurred only rarely, and that S. bovis derived genome blocks have been purified by ongoing hybridization with S. haematobium, the more relevant schistosome species naturally infecting humans. It was also hypothesized that hybridization was endemic and occurring where the cultural practices of humans and livestock sharing the same water bodies is common [5, 12-14].In Nigeria, nomadic farming and pastoralism are a common cultural practice, particularly in the northern regions of the country. Pastoral animals are grazed around rivers and streams which also serve as the main sources of water for the communities. Therefore, these regions exist as potential high-risk areas for hybridization between human and livestock schistosome species. To test this hypothesis, that interspecific S. haematobium hybrids pos- sessing a mixed ancestry with bovine schistosomes are common in water bodies shared between humans and cattle, we sampled two ecologically distinct S. haematobium endemic communities in Southwestern and Northern Nigeria. The sampled community in southern Nigeria is devoid of livestock whereas the community in the northern part of the country is pastoral. We report here the genetic profiles of the S. haematobium from the two locations using genetic markers anchored in the mitochondria (CO1, NAD5) and two unlinked nuclear genome markers (transITS, BF). The identification of homozygous alleles derived from animal schistosome species in sites devoid of livestock supports the notion that ongoing hybridization and backcrosses with animal schistosome species is oc- curring, independent of human-animal freshwater usage. Our work highlights the need to apply modern high throughput techniques to screen for hybrids to assess the degree to which ongoing hybridization is occurring be-tween animal and human schistosomes, and to identify the relevant hosts and infection reservoirs that are pro- moting these interactions.
Results and Discussion
Field Site data
Interspecific hybridization between animal and human schistosomes is thought to occur preferentially in pas- toral sites where animals and people share water bodies. To assess the degree to which this was occurring in Nigeria, two ecologically distinct S. haematobium endemic communities in southwestern and northern Nigeria were sampled (Figure 1).
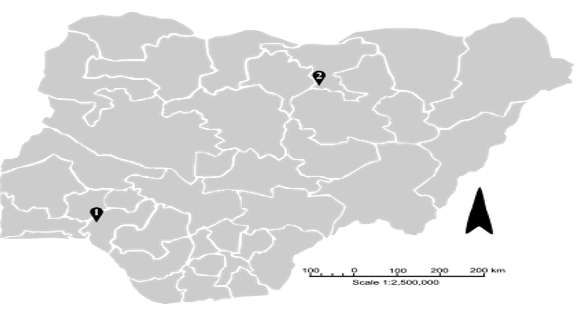
Figure 1. Map of Nigeria showing the 36 states and the specific sampling locations in Ondo State (1), Ile Oluji/OkeIgbo [latitude 5o 45N and 8o 15N and longitude 4o 30E], and Jigawa State (2), Kachi [latitude 11° 73' N and longi- tude 9° 33' E] for S. haematobium. Arrow depicts North.
In the community in southwest Nigeria that was devoid of livestock, 17 of 107 filters contained parasite eggs. DNA was extracted from these S. haematobium positive urine samples. Forty of 112 filters contained parasite eggs in the pastoral community in northern Nigeria, and DNA was extracted from these S. haematobium positive urine samples. In aggregate, the
prevalence of urogenital schistosomiasis infection was higher at the pastoral site in Kachi (35.7%) compared to the agrarian site in Ile Oluji/Oke Igbo (15.9%). The age of the participants ranged from 3-19 years, prevalence was higher in males (55.8%) than females (45.2%), but this did not reach significance (Table 1). Age was significantly associated with infection (p<0>(Table 1)
| Category | Ile Oluji/Oke Igbo | Kachi | Total | P value |
Human Infection | Negative | 90 (84.1) | 72 (64.3) | 162 (74.0) | 0.0008* |
| Positive | 17 (15.9) | 40 (35.7) | 57 (26.0) |
|
Infection Intensity | Heavy | 11 (64.7) | 17 (42.5) | 28 (49.1) | 0.1249 |
| Light | 6 (35.5) | 23 (57.5) | 29 (50.9) |
|
Gender | Female | 54 (50.5) | 45 (40.2) | 99 (45.2) | 0.1262 |
| Male | 53 (49.5) | 67 (59.8) | 120 (55.8) |
|
Age | < 10 | 73 (68.2) | 22 (19.6) | 95 (43.4) | <0> |
| > 10 | 34 (31.8) | 90 (80.4) | 124 (56.6) |
|
Table 1: Schistosoma haematobium prevalence in Ile Oluji/Oke Igbo (Ondo State) and Kachi (Jigawa State), Nigeria. Locations
The result of a cross between S. bovis x S. haematobium based on the two sequences obtained at the transITS marker on chromosome 2 (Table 2).
OD1 | IleOluji/Oke | Ondo | Human | S.h x S.c | S.h | S.b | S.b | |||
OD2 | IleOluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | S.b | S.b* | |||
OD3 | IleOlujOke | Ondo | Human | S.h x S.c | S.h | n.d. | S.b | |||
OD4 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h | S.b | S.b | |||
OD5 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h | S.b | S.b | |||
OD6 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | n.d. | S.b | |||
OD7 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h | S.b | S.b | |||
OD8 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | S.b | S.b | |||
OD9 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | S.b | S.b | |||
OD10 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | S.b | S.b | |||
OD11 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | S.b | S.b | |||
OD12 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | S.b | S.b | |||
OD13 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | S.b | S.b | |||
OD14 | Ile Oluji/Oke | Ondo | Human | S.c | S.h x S.c | S.b | S.b | |||
OD15 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | n.d. | S.b | |||
OD16 | Ile Oluji/Oke | Ondo | Human | S.h x S.c | S.h x S.b | S.b | S.b | |||
OD17 | Ile Oluji/Oke | Ondo | Human | S.c | S.h | S.b | S.b | |||
J1 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J2 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J3 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J4 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J5 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J6 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J7 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J8 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J9 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J10 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J11 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J12 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J13 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J14 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J15 | Kachi | Jigawa | Human | S.h x S.b* | S.h x S.b | S.h | S.h | |||
J16 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J17 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J18 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J19 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J20 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J21 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
| J22 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | ||
J23 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J24 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J25 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J26 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J27 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J28 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J29 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J30 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J31 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J32 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h* | |||
J33 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J34 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J35 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J36 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J37 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J38 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J39 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
J40 | Kachi | Jigawa | Human | S.h x S.b* | S.h | S.h | S.h | |||
| ||||||||||
Table 2: Isolate Location State Source Nuclear Markers Mitochondrial Markers
Of particular interest, a private SNP at nucleotide 502 (based on GenBank reference sequence MH014047) was identified that was unique to strains circulating in Kachi that has not been previously observed in any published S. haematobium or S. bovis allele (Suppl.Table 2).
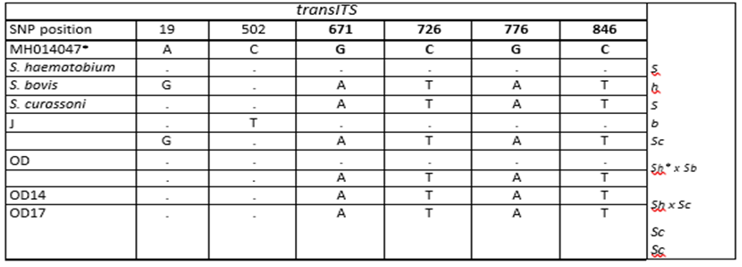
Supplementary Table 2: transITS haplotypes. * SNP position reference, S.haematobium NCBI (MH014047), J =Kachi Isolates, OD = Ile Oluji/Oke Igbo isolates, S.h = S. haematobium, S.b = S. bovisIt was not possible to interpret the origin of the private SNP, i.e., whether it was of S. bovis or S. haematobium ancestry. At CO1, all isolates had an S. haematobium haplogroup I allele (Figure 2).
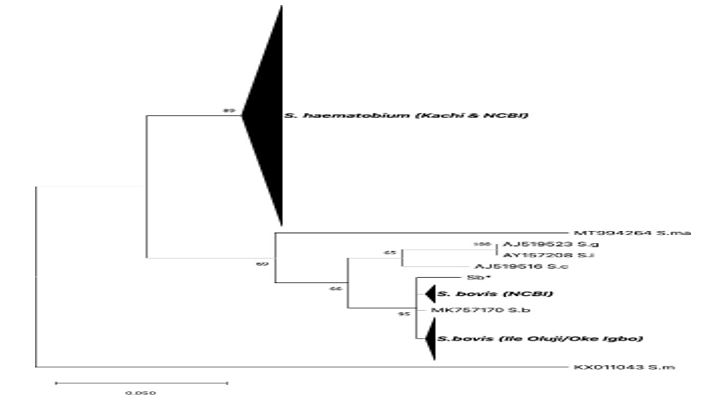
Figure 2. Maximum likelihood tree based on CO1 sequences obtained from S. haematobium isolates collected in Oluji/Oke Igbo and Kachi in Nigeria. Reference sequences from worms within the S.
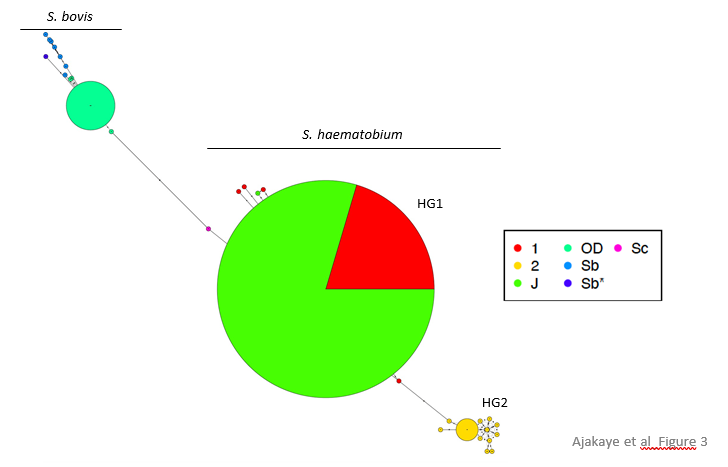
Haematobium species complex were added for comparative purposes, and are comprised of published sequences retrieved from Gen- Bank. The OC1 sequence from S. mansoni (KX011043) was used as the outgroup. (Sb* - this study, S. bovis worm from cow recovered from an abattoir in Nigeria); S.b = S. bovis; S.m = S. mansoni; S.c = S. curassoni; S.ma = S. mattheei; S.g = S. guineensis; S.i = S. intercalatum.One isolate possessed a single, unique (or private) SNP at CO1 indicating that more than one haplotype was circulating among the patient samples (Figure 3).
In Ile Oluji/Oke Igbo, an endemic site devoid of livestock, all patients were likewise infected with interspecific hybrids. At the CO1 marker, all isolates possessed alleles that branched with S. bovis (Table 2). Phylogenetic anal- ysis at CO1 showed that infected patients possessed one of 4 unambiguous sequences that branched separately, but clustered tightly with other S. bovis sequences, including those from a cow in Nigeria (this study) and from the public domain, all forming a monophyletic group that resolved from other human and livestock schistosomes within the S. haematobium species complex with good bootstrap support, including S. curassoni, S. mattheei, S. intercalatum, and S. guineensis (Figure 2).
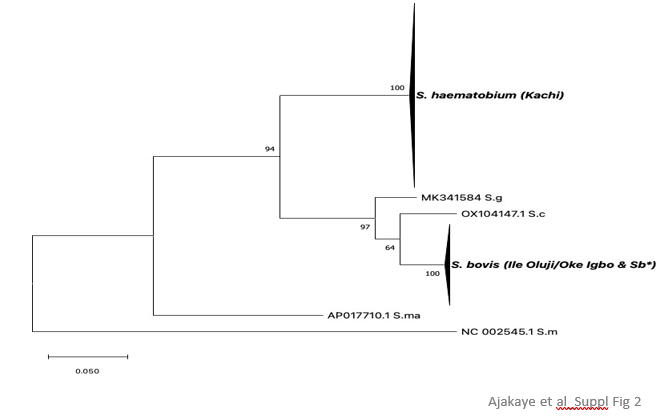
Suppl. Figure 2: Maximum likelihood tree at ND5. Phylogenetic analysis of ND5 alleles recovered from S. haemat-obium isolates in Oluji/Oke Igbo (OD) and Kachi, (J) in Nigeria. Only S. bovis alleles were identified among isolates recovered from Oluji/Oke Igbo (OD), whereas only S. haematobium alleles were identified among isolates recov-ered from Kachi, (J). * This study, (S. bovis worm from cow in Nigeria), S.g = S. guineensis, S.c = S. curassoni, S.m =S. mansoni
At CO1, one allele was dominant, and the other 3 alleles possessed a single, private SNP indicating that at least 4 haplotypes were circulating among the patient samples (Figure 3).
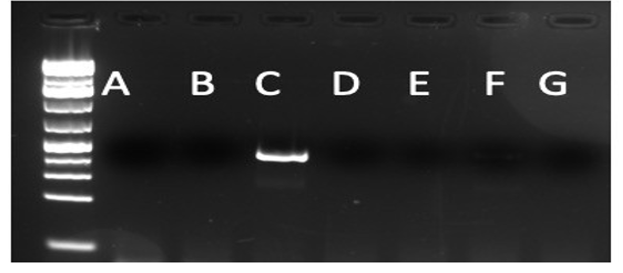
Suppl. Figure 3: ND5 S. bovis specific primer validation. New primers developed at ND5 were exclusively specific for S. bovis. An agarose gel stained with GelRed shows a PCR product only for the PCR reaction that used template DNA from S. bovis. Each well used the following template DNA for the PCR reaction: A (S.haematobium); B (S.mansoni); C (S. bovis); D (S. japonicum); E (S. mattheei); F (S. curassoni); G (Water)
Figure 3. Haplotype network at CO1. S. haematobium CO1 alleles circulating among isolates recovered from patients in Kachi, Jigawa state (J) belonged to the S. haematobium group 1 (HG1) haplotype (green). Red – S. haematobium haplogroup 1 reference sequences obtained from GenBank. For reference, S. haematobium group2 (HG2) haplotypes (yellow) are also depicted for sequences obtained from GenBank. S. bovis CO1 alleles circulat- ing among isolates recovered from patients in Oluji/Oke Igbo, Ondo state (OD) were distinct (seagreen) from se- quences obtained from GenBank annotated as S. bovis. Sb* represents the allele identified at CO1 for the S. bovis isolate recovered from an abattoir in Nigeria, that served as a control sequence for S. bovis. Further, mitonuclear discordance was observed in all 17 isolates, worms possessed an S. bovis mitochondrial an-cestry, but one of 2 different haplotypes based on the alleles present at the nuclear transITS marker, either ad- mixed (heterozygous between S. haematobium x S. curassoni) or homozygous for S. curassoni. The presence of livestock-specific schistosomes in two patients, the result of hybridization between S. bovis x S. curassoni with no evidence of S. haematobium was noteworthy but has been reported previously in Niger [22]. The other 15 isolates, however, were consistent with livestock:human schistosome hybrids. Our data show that a diverse array of hybrid livestock:human schistosomes are circulating in Nigeria, and they are causing infections in non-pastoral sites, de-void of livestock. Bravo-Figey (BF) domain-containing nuclear and ND5 mitochondrial markers.To better understand the true number of haplotypes present, and to further resolve the origin of the S. bovisx S. curassoni hybrids, we developed two additional genetic markers. The first, BF, a cell adhesion molecule (CAM)that belongs to the N-CAM transmembrane proteins, represents an unlinked marker located on chromosome 7. The gene has seven exons, is 4164 nucleotides in length, and PCR primers were designed in a nested configurationto amplify a polymorphic fragment within exon 2 that is 408 nucleotides in length. The genus-specific primers amplify all Schistosoma species with equal efficiency (data not shown). DNA sequencing was next used to distin-guish between the species, of which 29, 14, 9 and 3 SNPs separate the S. haematobium allele from that of S.mansoni, S. bovis, S. curassoni and S. mattheei reference sequences, respectively (Figure 4).
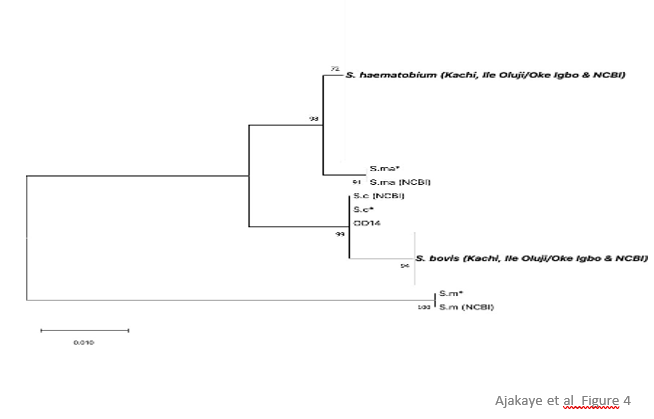
Figure 4. Maximum likelihood tree at BF. Phylogenetic analysis of BF alleles recovered from S. haematobium isolates in Oluji/Oke Igbo, Ondo state and Kachi, Jigawa state in Nigeria were compared against reference se-quences (S.m* = S. mansoni, S.ma* = S. mattheei, S.c* = S. curassoni and S. bovis). For isolates that were heterozygous, only non- S.haematobium alleles are shown in the tree. Hence, for sample OD14, only the S. curas-soni allele is depicted for the Sh x Sc alleles present at BF. Likewise, for OD2,6,8-13, 15-16, and J15, only the S. bovis allele is depicted for the Sh x Sb alleles present at BF. All other isolates were homozygous for S. haematobium alleles at BF.
In Kachi, all isolates but one possessed an unambiguous, homozygous S. haematobium BF allele whereas isolate J15 was heterozygous and had an S. bovis x S. haematobium allele (Table 2). In total, only two hybrid Sb x Sh haplotypes were resolved in Kachi among the 4 markers applied (Table 2). In contrast, in Ile Oluji/Oke Igbo, 3 allelic types were resolved atBF, either a homozygous S. haematobium allele in 6 of 17 isolates, a heterozygous admix of S. haematobium x S. bovis alleles in 10 of 16 isolates, and one heterozygous admix of S. haematobium x S. curassoni in the final isolate (Table 2). Importantly, in the two isolates (OD14, OD17) that represented Sb x Sc livestock: livestock hybrids when assessed at just CO1 and ITS genotyping markers, inclusion of the BF marker established that the genotypes for these two isolates were hybrid livestock (Sb x Sc) by human (Sh) recombinants that had undergone repeated in- terspecific hybridizations, including at least two separate backcrosses, in the origin of these human infective schis- tosomes. For the majority of the other Ile Oluji/Oke Igbo isolates, a cross between a S. haematobium male with a hybrid S. bovis x S. curassoni female would be sufficient to produce the genotype resolved, specifically a maternally inherited S. bovis mitochondrial genome that is heterozygous with an Sh and either an Sb or Sc allele at the nuclear markers. Further, in this agrarian community, in the absence of livestock, it may also suggest that people are playing an important role in the origin and evolution of these human infective hybrids. It will be important to genotype individual miracidia isolated from patient samples to determine the extent to which mixed infectionsare occurring that could promote cross-pairing among the various species within the S. haematobium speciescomplex. Finally, recombination and rapid in situ evolution has been shown to occur in mitochondrial genomes for a variety of parasitic pathogens during interspecific mating [23] or during passage [24]. We developed PCR primers (genus- and S. bovis- specific primers; Suppl. Fig 3) in a nested configuration to amplify the ND5 gene within the mitochondrial genome. The genus-specific primers amplified all Schistosoma species with equal efficiency (data not shown). When applied against all isolates, no interspecific recombination was resolved, and the ND5 sequence types were in linkage with the sequence type resolved at CO1 confirming that the isolates from Kachi had inherited an S. haematobium mitochondrial genome, and those from Ile Oluji/Oke Igbo had inherited an S. bovis mitochondrial genome (Table 2). Two isolates, one each from Kachi and Ile Oluji/Oke Igbo, each possessed a single private SNP, increasing the number of haplotypes resolved among the patient samples (Suppl. Fig. 2). Ile Oluji/Oke Igbo.
Materials and Methods
Ethics Statement
This study was conducted under the National ethical permit and protocol numbers NHREC/01/01/2007-30/10/2020 and NHREC/01/01/2007- 29/03/2022B. After informed consent by parents and guardians, about 20ml of urine was collected in specimen bottles from a total number of 219 participants ranging in age from four to fourteen years.
Study sites
Ile Oluji/Oke Igbo is an administrative area of Ondo state situated in the tropical rainforest belt of Southwest-ern Nigeria. The area which lies between latitude 5o 45N and 8o 15N and longitude 4o 30E is made up of fourteen villages. The climate is humid with small seasonal and daily variations in rainfall. The rainfall is concentrated during the months of May to October with a short break in August and considerable variations from year to year. The area is surrounded by many rivers, such as Owena, Aigo, Esinmu, Iyire, Ogburu, Oni and Awo. The inhabitants ofthe study area are primarily farmers, engaging in small to medium scale production of both cash and arable crops like yam, cassava, kolanut, cocoa and maize among others [15] (Fig. 1). Kachi is a village situated in Northern Nigeria under Dutse Local Government Area of Jigawa State (latitude 11° 73' N and longitude 9° 33' E ). Dutse is a city located in Northern Nigeria and is the capital of Jigawa State. The population is predominantly the Hausa and Fulani tribes practicing agriculture and pastoralism as the main source of livelihood. The climate of the area is tropical wet and dry, and the vegetation type is Sudan Savannah despite its rocky topography typical of Dutse. Over the course of the year, the temperature typically varies reaching highs of 39°C [16] (Figure 1).
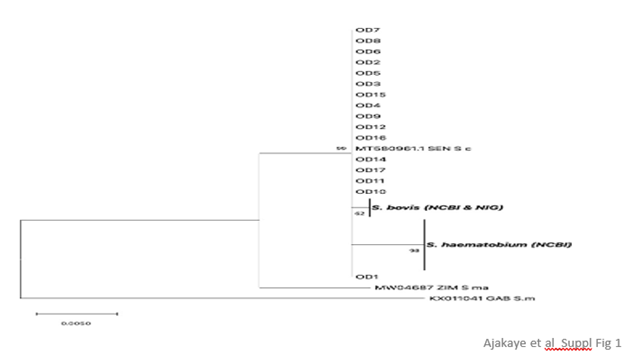
Supplementary Figure 1. Maximum likelihood tree at the transITS marker for S. haematobium isolates in Oluji/Oke Igbo (OD). For isolates OD14 and OD17, only an S. curassoni allele was recovered at transITS. All other isolates were heterozygous and possessed an S. haematobium allele x S. curassoni allele. Only the S. curassoni allele from OD is depicted for comparison against reference alleles for the following: S. bovis Nig = S. bovis worm recovered from a cow in Nigeria. S. bovis (NCBI) = MW027649.1, MT158872.1, MW027648.1, MF776588.1, 416 MF776589.1. S. haematobium (NCBI) = MH014047, GU257398, JQ397400, JQ397401, JQ397402, JQ397403, 417 JQ397404, JQ397405, JQ397406, JQ397407, JQ397408, JQ397409, JQ397410, JQ397411, JQ397412, JQ397413, JQ397414. S.c = S. curassoni isolate from SEN (Senegal), GenBank Accession number MT580961. S. ma = S. mat-theei isolate from ZIM (Zimbabwe), GenBank Accession number MW04687. S. m = S. mansoni isolate from GAB (Gabon), GenBank Accession number KX011041.
Parasitological analysis
Urine (20ml) was collected in specimen bottles labeled with a unique identifier number on a pre-designed epidemiological form. The sex and age of the participants was entered against the appropriate number on the epidemiological form when urine samples were submitted. In the laboratory, identification of S. haematobium eggs was based on their characteristic terminal spine, and both the prevalence and infection intensity were cal- culated by microscopy. Infection intensity was determined as the number of eggs detected per 10 ml of urine (eggs/10 ml). Light infection was categorized as 1–49 eggs/10 ml and heavy infections ≥50 eggs/10 ml [17]. Urine positive samples for S. haematobium eggs were subsequently filtered using Sterlitech 13 mm polycarbonate screen membrane filters (https://www.sterlitech.com/schistosome-test-kit.html) for collection of eggs and pre-served in absolute ethanol for molecular analysis.
Molecular analysis
Total DNA was extracted from individual filters and five Schistosoma species were used as positive controls (S. mansoni, S. curassoni, S. bovis, S. mattheei, and S. haematobium) using QAIGEN Blood and Tissue kit following the manufacturers protocol (www.qiagen.com). The filters were incubated at 56℃ in lysis buffer overnight prior to completing the DNA isolation. Isolated DNA was eluted in molecular grade water and stored at -20℃. Partialregion of the mitochondrial cytochrome oxidase subunit 1 (CO1) and the complete nuclear ribosomal internal transcribed spacers (transITS) gene regions were amplified for the isolates using published primers Cox1_schist_5ʹ(5ʹTCTTTRGATCATAAGCG3ʹ) Cox1_schist_3ʹ(5ʹTAATGCATMGGAAAAAAACA-3ʹ) andETTS1(5'TGCTTAAGTTCAGCGGGT-3'), ETTS2 (5'TAACAAGGTTTCCGTAGGTGAA-3'), respectively [18, 19]. We designed new pan-genus primers targeting the mitochondrial Nad5 gene (5ʹ-GGGTAAAAGTTGGAATTTGAGGG-3ʹ and 5ʹ-CGCTTTAACCATCTGACCACC-3’) and an S. bovis-specific (5ʹ TCGATTTGGAGATGTGGCGT-3ʹ and 5ʹ-ACTGAACTAAA- GCCAAGTCTACC-3ʹ) primer to provide confirmatory data sets for validating Cox1 data sets that do not possess an S. haematobium allele. We also identified a new nuclear gene marker, Bravo_Figey domain containing protein gene (BF) and designed a nested primer set (EXT:5ʹ TGTATCACGCTGGCCATACT-3ʹand 5ʹ-CCAC- CTGCCATCAAACTCAC-3’; INT: 5ʹ-ACTAGATGGCAGATACGGACC-3ʹ and 5ʹ-TAGTCCCCTTGAGGTTGTCG-3’) to amplify the polymorphic region between S. haematobium and S. bovis. We also sequenced DNA from our five other ref-erence Schistosome species to obtain sequences for phylogenetic analysis. We used the following PCR cycling conditions for the NAD5 and BF primers, 3 minutes at 98°C followed by 35 cycles each of 20 seconds at 98°C, 15 seconds at 58°C followed by 30 seconds at 72°C, and a final elongation step at 72°C for 1 minute. All PCRs were performed in a 25ul reaction using KAPA HIFI Taq reagents, 0.25ul of 50um forward and reverse primers and 1ul of DNA. 4ul of amplicons was visualized on agarose gel by gel electrophoresis. Amplicons were purified using Ampure XP beads and Sanger sequenced in both directions.
Genetic analysis
All sequences were imported into Geneious vs 2022.2.1 for de novo assembly and trimming.
Polymorphic
positions in consensus sequences were crosschecked by visualizing the original chromatograms of the forward and reverse sequences. The NCBI nucleotide blast tool (https://blast.ncbi.nlm.nih.gov) was used to initially con-firm the identity of each consensus sequence. Several published Schistosoma species sequences were retrieved from the NCBI nucleotide database (Suppl. Table1)
Haplotype | S. h HG 1 | S. h HG2 | J (Kachi) | OD (Ile Oluji/Oke Igbo) | S. bovis | S. b * (S. bovis ,NIG) | S. curassoni | NCBI Sequences in Haplogroups |
I | 10 | 0 | 39 | 0 | 0 | 0 | 0 | GU257337,JQ397332,JQ397345,JQ397350,JQ397351,JQ397352,JQ397353,JQ397397,JQ397369,JQ397392 |
II | 1 | 0 | 0 | 0 | 0 | 0 | 0 | JQ397349 |
III | 1 | 0 | 0 | 0 | 0 | 0 | 0 | JQ397368 |
IV | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
|
V | 1 | 0 | 0 | 0 | 0 | 0 | 0 | JQ082121 |
VI | 1 | 0 | 0 | 0 | 0 | 0 | 0 | JQ397393 |
VII | 0 | 1 | 0 | 0 | 0 | 0 | 0 | JQ397399 |
VIII | 0 | 5 | 0 | 0 | 0 | 0 | 0 | GU257334,GU257338,GU257348,GU257360,JQ397398 |
IX | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257346 |
X | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257336 |
XI | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257335 |
XII | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257345 |
XIII | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257352 |
XIV | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257354 |
XV | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257356 |
XVI | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257358 |
XVII | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257342 |
XVIII | 0 | 1 | 0 | 0 | 0 | 0 | 0 | GU257349 |
XIX | 0 | 0 | 0 | 0 | 0 | 0 | 1 | AJ519516 |
XX | 0 | 0 | 0 | 0 | 1 | 0 | 0 | MW022138 |
XXI | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
|
XXII | 0 | 0 | 0 | 0 | 1 | 0 | 0 | AJ519521 |
XXIII | 0 | 0 | 0 | 0 | 1 | 0 | 0 | MT579448 |
XXIV | 0 | 0 | 0 | 11 | 0 | 0 | 0 |
|
XXV | 0 | 0 | 0 | 0 | 1 | 0 | 0 | MF919415 |
XXVI | 0 | 0 | 0 | 0 | 1 | 0 | 0 | MK757170 |
XXVII | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
|
XXVIII | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
|
XXIX | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
|
XXX | 0 | 0 | 0 | 0 | 1 | 0 | 0 | AY157212 |
XXXI | 0 | 0 | 0 | 0 | 1 | 0 | 0 | FJ897160 |
Supplementary Table 1: CO1 haplotypes based on Fig 3
for phylogenetic and network analysis with the novel sequence datasets from this study (Table 2). All sequences were aligned using MUSCLE program within the Geneious soft- ware prior to subsequent analysis. The molecular phylogenetic placement of the sequence data was implemented in MEGA v11 [20], using Maximum Likelihood method at 1000 bootstrap replicates to ascertain the reliability of the tree branches. Haplotype network was implemented in an R environment using the Pegas package [21].
Conclusion
It is apparent that the commonly used CO1 and ITS gene markers are inadequate for correctly characterizing or categorizing S. haematobium isolates as either pure or hybrid. Therefore, there is a need to develop more markers and apply them using modern high throughput methods to more precisely determine the extent and number of circulating interspecific hybrids to prioritize these samples for whole genome sequencing. We propose a multilocus typing scheme involving linked and unlinked nuclear gene markers located on the seven S.haematobium chromosomes to determine the extent of hybridization that is occurring in schistosomiasis-endemic settings. We also provide compelling evidence that admixed S. haematobium:livestock schistosomes are widespread and their origin and transmission may be less dependent on human/cattle contact than previously envisaged. Investigating the genetic diversity and degree of interspecific S. haematobium hybrids circulating in snail vector and other animal reservoir hosts (for example, rodents) may provide useful insight in the transmission and origin of these interspecific hybrids. It will also be important to individually genotype individual miracidia derived from infected individuals to assess the degree of mixed infections, and to assess whether cross-pairings with animal:human schistosomes is occurring in situ in infected people.
Finally, there is an urgent need to identify a ‘pure’ S. haematobium reference sequence (if it exists). It will be important to screen isolates from Zanzibar or Madagascar where no S. bovis ancestry has been recorded. This will provide the genomic backbone to compare whole genome sequences of S. haematobium populations from different geographical regions, in order to map introgressed and genomic regions that are promoting disease transmission and host range specificity.
Acknowledgements
We would like the thank the Natural History Museum, London for the genetic material provided via the Schisto- somiasis Collection at the Natural History Museum (SCAN) archive, it was curated and collected in a collaborative effort, and we acknowledge the support and generosity of the partnerships involved in the collections, particularly in relation to the endemic countries involved, and past colleagues that maintained the live material. The Schisto-somiasis Collection at the Natural History Museum (SCAN) was funded by the Wellcome Trust (grant 104958/Z/14/Z). We would also like to thank the Schistosome Resource Centre of the Biomedical Research Insti-tute (sponsored by NIAID, NIH), and in particular, Dr. Margaret Mentink-Kane.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supporting information
All supporting information can be found in the Supplementary Figures and Tables provided.
Financial Disclosure Statement
This work was supported by an International Foundation of Science (IFS) grant I3-B-6522-1 and by the Division of Intramural Research within the National Institute of Allergy and Infectious Diseases (NIAID)at the National Institutes of Health (NIH).
Author contributions
AOG-Conceptualization, Formal Analysis, Data Curation, Visualization, Original Draft Preparation
EEE- Formal Analysis, Original Draft Preparation
BJB- Investigation
OOT- Investigation
GME- Data Curation, Supervision, Validation, Review & Editing, Resources
References
- Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6(7):411-425.
View at Publisher | View at Google Scholar - Utzinger J, Raso G, Brooker S, et al. Schistosomiasis and neglected tropical diseases: towards integrated and sustainable control and a word of caution. Parasitology. 2009;136(13):1859-1874.
View at Publisher | View at Google Scholar - Enabulele EE, Platt RN, Adeyemi E, et al. Urogenital schistosomiasis in Nigeria post receipt of the largest single praziquantel donation in Africa. Acta Trop. 2021; 219:105916.
View at Publisher | View at Google Scholar - Adeyemo P, Leger E, Hollenberg E, et al. Estimating the financial impact of livestock schistosomiasis on traditional subsistence and transhumance farmers keeping cattle, sheep and goats in northernSenegal. Parasit Vectors. 2022;15(1):101.
View at Publisher | View at Google Scholar - Huyse T, Webster BL, Geldof S, et al. Bidirectional introgressive hybridization between cattle and human schistosome species. PLoS Pathog. 2009;5(9): e1000571.
View at Publisher | View at Google Scholar - Rey O, Webster BL, Huyse T, et al. Population genetics of African Schistosoma species. Infect Genet Evol.2021;89:104727.
View at Publisher | View at Google Scholar - Onyekwere AM, Rey O, Allienne JF, et al. Population Genetic Structure and Hybridization of Schistosoma haematobium in Nigeria. Pathogens. 2022;11(4):425.
View at Publisher | View at Google Scholar - Platt RN, McDew-White M, Le Clec'h W, et al. Ancient Hybridization and Adaptive Introgression of an Invadolysin Gene in Schistosome Parasites. Mol Biol Evol. 2019;36(10):2127-2142.
View at Publisher | View at Google Scholar - Rey O, Toulza E, Chaparro C, et al. Diverging patterns of introgression from Schistosoma bovis across S. haematobium African lineages. PLoS Pathog. 2021;17(2): e1009313.
View at Publisher | View at Google Scholar - Kincaid-Smith J, Tracey A, de Carvalho Augusto R, et al. Morphological and genomic characterisation of the Schistosoma hybrid infecting humans in Europe reveals admixture between Schistosoma haematobium and Schistosoma bovis. PLoS Negl Trop Dis. 2021;15(12): e0010062.
View at Publisher | View at Google Scholar - Oey H, Zakrzewski M, Gravermann K, et al. Whole-genome sequence of the bovine blood fluke Schistosoma bovis supports interspecific hybridization with S. haematobium. PLoS Pathog. 2019;15(1): e1007513. Published 2019 Jan 23.
View at Publisher | View at Google Scholar - Fall CB, Lambert S, Léger E, et al. Hybridized Zoonotic Schistosoma Infections Result in HybridizedMorbidity Profiles: A Clinical Morbidity Study amongst Co-Infected Human Populations of Senegal. Microorganisms. 2021;9(8):1776. Published 2021 Aug 20.
View at Publisher | View at Google Scholar - Leger E, Borlase A, Fall CB, et al. Prevalence and distribution of schistosomiasis in human, livestock, and snail populations in northern Senegal: a One Health epidemiological study of a multi-host system. LancetPlanet Health. 2020;4(8): e330-e342.
View at Publisher | View at Google Scholar - Borlase A, Rudge JW, Léger E, et al. Spillover, hybridization, and persistence in schistosome transmission dynamics at the human-animal interface. Proc Natl Acad Sci U S A. 2021;118(41): e2110711118.
View at Publisher | View at Google Scholar - Ondo State Profile.
View at Publisher | View at Google Scholar - Dutse Emirate. Available from https://www.jigawastate.gov.ng/dutse.php. (Accessed 23 July 2022)
View at Publisher | View at Google Scholar - Montresor A, Crompton DWT, Hall A, Bundy DAP, Savioli L. Guidelines for the evaluation of soil-transmitted helminthiasis and schistosomiasis at community level: a guide for managers of control programmes, 1988. WHO.
View at Publisher | View at Google Scholar - Lockyer AE, Olson PD, Ostergaard P, et al. The phylogeny of the Schistosomatidae based on three genes with emphasis on the interrelationships of Schistosoma Weinland, 1858. Parasitology. 2003;126(Pt 3):203-224.
View at Publisher | View at Google Scholar - Kane RA, Rollinson D. Repetitive sequences in the ribosomal DNA internal transcribed spacer of Schistosoma haematobium, Schistosoma intercalatum and Schistosoma mattheei. Mol BiochemParasitol. 1994;63(1):153-156.
View at Publisher | View at Google Scholar - Tamura K, Stecher G, Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol BiolEvol. 2021;38(7):3022-3027.
View at Publisher | View at Google Scholar - Paradis
View at Publisher | View at Google Scholar - Leger E, Garba A, Hamidou AA, et al. Introgressed Animal Schistosomes Schistosoma curassoni and S. bovis Naturally Infecting Humans. Emerg Infect Dis. 2016;22(12):2212-2214.
View at Publisher | View at Google Scholar - Romano A, Inbar E, Debrabant A, et al. Cross-species genetic exchange between visceral and cutaneous strains of Leishmania in the sand fly vector. Proc Natl Acad Sci U S A. 2014;111(47):16808-16813.
View at Publisher | View at Google Scholar - Kinkar L, Gasser RB, Webster BL, et al. Nanopore Sequencing Resolves Elusive Long Tandem-Repeat Regions in Mitochondrial Genomes. Int J Mol Sci. 2021;22(4):1811. Published 2021 Feb
View at Publisher | View at Google Scholar

 Clinic
Clinic
