Review Article | DOI: https://doi.org/10.31579/2834-5029/011
A Concise Review- An Analytical Method Development and Validation of Armodafinil
- Pritam Jain *
- Manali Bhamre
- Mayur Nandre
- Snehal Dhulgunde
Patel Institute of Pharmaceutical Education and Research, Karwand Naka, Shirpur.
*Corresponding Author: Pritam Jain, R.C. Patel Institute of Pharmaceutical Education and Research, Karwand Naka, Shirpur.
Citation: Ashwin Singh Chouhan. (2023), A Concise Review- An Analytical Method Development and Validation of Armodafinil, International Journal of Biomed Research. 2(1): DOI: 10.31579/2834-5029/011
Copyright: Ashwin Singh Chouhan. (2023), A Concise Review- An Analytical Method Development and Validation of Armodafinil, International Journal of Biomed Research. 2(1): DOI: 10.31579/2834-8087/011
Received: 05 January 2023 | Accepted: 30 January 2023 | Published: 06 February 2023
Keywords: RP-HPLC; armodafinil; method development and validation
Abstract
The HPLC method for Armodafinil both bulk & in combination are given in Table 1 which includes parameters like matrix, stationary phase, mobile phase composition, detection wavelength RF value, retention time etc. HPTLC method reported in Table 2 includes parameter like matrix, stationary phase, mobile phase, RF, DL etc. The table 3 includes the GC-MS method for Armodafinil which involve the parameters like Matrix, stationary phase, mobile phase composition, Carrier gas, Retention time, flow rate etc. The table 4 includes the Capillary Electrophoresis method for Armodafinil which involve the parameters like Matrix, Capillaries wavelength, Separation Voltage, Temperature and pressure etc. Spectrometric methods for Armodafinil include UV-Visible Spectroscopy.
Introduction
Armodafinil is the R-enantiomer of modafinil, a wake-promoting drug that predominantly affects brain areas involved in wakefulness control [1]. The US Food and Medicine Administration has licensed the drug for the treatment of individuals with excessive drowsiness caused by obstructive sleep apnea, narcolepsy, or shift work disorder [2]. The working mechanism is still a mystery. The dopamine transporter in the striatum and the norepinephrine transporter in the thalamus are both sensitive to modafinil [3]. Hypocretin, histamine, -adrenergic, -aminobutyric acid and/or glutamate receptors are all affected by modafinil [4]. 2-[(R)-(diphenylmethyl) sulfinyl] acetamide and 2-(R-benzhydrylsulfinyl) acetamide are the chemical names for armodafinil [5].
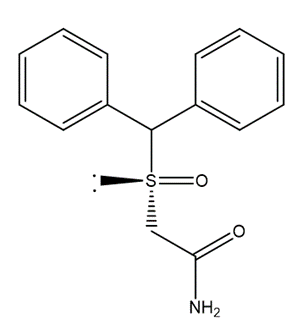
Armodafinil Pharmacodynamics:
Modafinil and its R-enantiomer, armodafinil, have uncertain therapeutic mechanisms in vivo [6]. Armodafinil inhibits dopamine re-uptake via binding to the dopamine transporter. It is not, however, a dopamine receptor agonist that acts directly or indirectly. In some animal brain regions, these binding inhibitory effects have been linked to higher extracellular dopamine levels [5]. Modafinil has complicated pharmacodynamic features since it interacts with a number of central pathways, including the catecholaminergic system. Both the R- and S-enantiomers bind to DAT35 and raise DA levels in many brain locations, including the prefrontal cortex (PCF), enhancing executive functions such as attention, impulse control, and memory [7].
Amodafinil pharmacokinetics:
Absorption: After numerous or a single oral administration, modafinil is absorbed at least 40 to 65 percent (oral bioavailability) and reaches maximum plasma concentrations (Cmax) 2–4 hours later. Because of its limited water solubility, it is not suitable for intravenous delivery in humans [7]. Oral administration of armodafinil causes rapid absorption, with peak plasma concentrations appearing in about 2 hours in the fasting condition. Food has no effect on armodafinil's overall bioavailability; however, the time to peak concentration can be delayed by 2–4 hours [5].
Distribution: Modafinil has a plasma protein binding of around60%, primarily to albumin, and an apparent volume of distribution of 0.8 L/kg following single or several oral doses, indicating that it can easily permeate tissues [7]. About 60% of modafinil is linked to plasma proteins, primarily albumin [8].
Metabolism: Modafinil is extensively degraded in the liver, largely via amide hydrolysis to form an acid metabolite, into inactive metabolites; ((±)2- [(diphenylmethyl) sulfinyl] acetic acid; modafinic acid) catalyzed by an esterase and/or amidase; ii) by S-oxidation via cytochrome CYP3A4 or CYP3A5 to produce a sulfone (2- [(diphenylmethyl) sulfanilyl] acetamide); iii) by aromatic ring hydroxylation; and iv) by glucuronide conjugation [7]. The principal metabolic process is amide hydrolysis, which does not require cytochrome P450 (CYP) activity. Cytochrome CYP3A4/5 plays a role in sulfone production [8].
Excretion: The elimination half-life is roughly 12–15 hours, owing to the kinetics of the R-enantiomer, as the S-enantiomer has a half-life of 4–5 hours [9]. Individuals with cirrhosis had a 60% reduction in modafinil clearance, while patients with chronic hepatic insufficiency have a doubled Cmax [10]. The main urinary metabolite, modafinil acid, accounts for 35 percent to 60 percent of the dosage [11].
Analytical accounts on Armodafinil:
The widespread literature survey exposed multiple analytical techniques like UV spectrophotometry method, HPLC, HPTLC, LC-MS/MS, for the determination of Armodafinil in bulk and pharmaceutical formulation. These reported methods describe the evaluation of armodafinil in various dosage forms like tablets and matrix like human plasma.
Chromatographic overview:
HPLC Method
P. Vivek Sagar et. al. outlined a stability showing RP HPLC method for the estimation of armodafinil in tablet dosage form. Chromatography was carried out using isocratic elution on a 4.6 x 250 mm stainless steel Hibar C18 column filled with octadecylsilane bound to porous silica (C18) with a particle size of 5 micron. The mobile phase is made up of 50:50 v/v acetonitrile and water. The effluent is measured at 220 nm and the flow rate is 1.0 ml/min. The retention time for armodafinil was 3.8 minutes [12].
Kambham Venkateswarlu et. al. given a validated stability indicating RP-HPLC method for estimation of Armodafinil in pharmaceutical dosage forms; also presented characterization of its base hydrolytic product. The separation was carried out on a C18 column with a 45:55 percent v/v combination of water and methanol as the mobile phase. At 1ml/min, eluents were identified at 220nm. Milder stress conditions were used first, followed by greater circumstances. For Armodafinil, the linearity of the suggested approach was tested in the range of 20-120g/ml. It was discovered that the retention time was 8.1 minutes [13].
Devi Ramesh et. al. performed an analytical approach for development and validation of new LC-MS/MS method for the determination of armodafinil in human plasma. Using 0.2 percent formic acid: methanol (15:85 v/v) as mobile phase on a hypurity advance C-18 column (5; 100 4.6 mm) at a flow rate of 1.0 ml/min, chromatographic separation was obtained in 3.0 minutes. The linearity of the drug concentration range of 50-10000 ng/mL was demonstrated (r2 = 0.9989) [14].
Ramisetti Nageswara Rao et. al. given an enantioselective HPLC resolution of synthetic intermediates of armodafinil and related substances; where armodafinil was studied on polysaccharide-based stationary phases, viz. cellulose tris-(3,5-dimethylphenylcarbamate) (Chiralcel OD-H) and amylose tris-(3,5-dimethylphenylcarbamate) (Chiralpak AD-H) by HPLC. When comparing the cellulose-based Chiralcel OD-H column to the amylose-based Chiralpak AD-H column, a satisfactory separation was achieved. A mobile phase containing n-hexane–ethanol–TFA (75:25:0.15 v/v/v) was used to achieve baseline separation with Rs A1.38. At 225 nm, a photodiode array detector was used to detect the enantiomers, while a polarimetric detector was used to identify the enantiomers [15].
CN Prathyusha Naik et. al. performed stability indicating assay method of armodafinil. The C8 (250 x 4.6 mm, 5m) column was used to separate the mobile phase of water and methanol (10 percent v/v OPA) 55:45 percent v/v. At 1ml/min, eluents were identified at 225 nm. Stress tests were carried out utilising acid, base, oxidizing agents, light, and heat to achieve a 10-20
Spectrophotometric overview:
UV-Visible Spectroscopy Method:
Tejaswi Jonnalagadda et. al. reported a simple visible spectrophotometric method for the determination of armodafinil in bulk and pharmaceutical dosage form. In the range of 10- 50 g/ml, the drug follows Beer Lambert law, with a correlation coefficient of 0.999. Armodafinil's percentage recovery in pharmaceutical dosage form is between 96 and 106 percent. The oxidative coupling reaction of 3-methyl-2-benzathiazoline hydrazone (MBTH) in the presence of ferric chloride is the basis for this approach (Fecl3). With the solvent system methanol: water, an absorption-maxima were discovered at 596nm [29].
Financial Disclosure Statement:
No funding to disclose.
Competing Interests Statement:
No competing interests to declare.
References
- Garnock-Jones, K. P., Dhillon, S. and Scott, L.J., (2009). Armodafinil. CNS drugs, 23(9), pp. 793-803.
View at Publisher | View at Google Scholar - Bogan, R.K., (2010). Armodafinil in the treatment of excessive sleepiness. Expert opinion on pharmacotherapy, 11(6), pp.993-1002.
View at Publisher | View at Google Scholar - Wittkampf, L.C., Arends, J., Timmerman, L. and Lancel, M., (2012). A review of modafinil and armodafinil as add-on therapy in antipsychotic-treated patients with schizophrenia. Therapeutic Advances in Psychopharmacology, 2(3), pp.115-125.
View at Publisher | View at Google Scholar - Loland, C.J., Mereu, M., Okunola, O.M., Cao, J., Prisinzano, T.E., et al, (2012). R-modafinil (armodafinil): a unique dopamine uptake inhibitor and potential medication for psychostimulant abuse. Biological psychiatry, 72(5), pp.405-413.
View at Publisher | View at Google Scholar - Lankford, D.A., (2008). Armodafinil: a new treatment for excessive sleepiness. Expert opinion on investigational drugs, 17(4), pp.565-573.
View at Publisher | View at Google Scholar - J. Kinslowa, Steven D. Shapirob, Michael F. Grunebaumc, Eliza C. Miller, Acute hypertensive crisis and severe headache after concurrent use of armodafinil and tranylcypromine: Case report and review of the literature Connor.
View at Publisher | View at Google Scholar - Sousa, A. and Dinis-Oliveira, R.J., (2020). Pharmacokinetic and pharmacodynamic of the cognitive enhancer modafinil: relevant clinical and forensic aspects. Substance Abuse, 41(2), pp.155-173.
View at Publisher | View at Google Scholar - Niemegeers, P., Maudens, K.E., Morrens, M., Patteet, L., Joos, L., et al, (2012). Pharmacokinetic evaluation of armodafinil for the treatment of bipolar depression. Expert opinion on drug metabolism & toxicology, 8(9), pp.1189-1197.
View at Publisher | View at Google Scholar - Kumar, R., (2008). Approved and investigational uses of modafinil. Drugs, 68(13), pp.1803-1839.
View at Publisher | View at Google Scholar - Dinges, D.F., Arora, S., Darwish, M. and Niebler, G.E., (2006). Pharmacodynamic effects on alertness of single doses of armodafinil in healthy subjects during a nocturnal period of acute sleep loss. Current medical research and opinion, 22(1), pp.159-167.
View at Publisher | View at Google Scholar - Wong, Y.N., King, S.P., Simcoe, D., Gorman, S., Laughton, W., et al, (1999). Open‐label, single‐dose pharmacokinetic study of modafinil tablets: influence of age and gender in normal subjects. The Journal of Clinical Pharmacology, 39(3), pp.281-288.
View at Publisher | View at Google Scholar - Sagar, P.V., Bagum, N. and Rani, S.S., (2014). Stability indicating RP HPLC method for the estimation armodafinil in tablet dosage form. Int. J. Pharm. Pharma Sci, 6, pp.604-609.
View at Publisher | View at Google Scholar - Venkateswarlu, K., Rangareddy, A., Narasimhaiah, K., Sharma, H., Mallikarjuna, N. et al, (2017). A validated stability indicating RP-HPLC method for estimation of Armodafinil in pharmaceutical dosage forms and characterization of its base hydrolytic product. Pak J Pharm Sci, 30(1), pp.23-8.
View at Publisher | View at Google Scholar - Ramesh, D., Ramakrishna, S. and Habibuddin, M., (2012). Development and Validation of New LC-MS/MS Method for the Determination of armodafinil in Human Plasma. Current Pharmaceutical Analysis, 8(3), pp.295-305.
View at Publisher | View at Google Scholar - Nageswara Rao, R., Shinde, D.D. and Kumar Talluri, M.V., (2008). Enantioselective HPLC resolution of synthetic intermediates of armodafinil and related substances. Journal of separation science, 31(6‐7), pp.981-989.
View at Publisher | View at Google Scholar - Naik, C.P. and Sekhar, K.C., (2018). Reliable and Sensitive Stability Indicating Assay Method of Armodafinil. Journal of Young Pharmacists, 10(1), p.52.
View at Publisher | View at Google Scholar - Jain, D. and Basniwal, P.K., (2016). Intrinsic stability study of armodafinil hydrochloride by forced degradation and impurity profiling. Pharm Anal Acta, 7, p.466.
View at Publisher | View at Google Scholar - Nagappan, K.V., Sungroya, N., Devi, D., Yamjala, K., Byaran, G. et al, (2017). Development and Validation of Stability Indicating RP HPLC method for the estimation of armodafinil and Characterization of its base Degradation Product by LC-MS/MS. GSTF Journal of Advances in Medical Research, 2(1).
View at Publisher | View at Google Scholar - Khile, A.S., Devi, N.G., Rao, M.S. and Ramachandran, D., (2017). Development And Validation Of RP-LC Method For Armodafinil in Pharmaceutical Formulations.
View at Publisher | View at Google Scholar - Chandasana, H., Kast, J., Bittman, J.A. and Derendorf, H., (2018). Quantitative determination of armodafinil in human plasma by liquid chromatography–electrospray mass spectrometry: Application to a clinical study. Biomedical Chromatography, 32(11), p.e4342.
View at Publisher | View at Google Scholar - Ramesh, D. and Habibuddin, M., Application of Validated Rp-Hplc Method for The Determination of Armodafinil in Bulk and Formulation. Int J Curr Pharm Res, 9(5), pp.158-161.
View at Publisher | View at Google Scholar - Harvanová, M. and Gondová, T., (2017). New enantioselective LC method development and validation for the assay of modafinil. Journal of Pharmaceutical and Biomedical Analysis, 138, pp.267-271.
View at Publisher | View at Google Scholar - Nageswara Rao, R., Shinde, D.D. and Kumar Talluri, M.V., (2008). Enantioselective HPLC resolution of synthetic intermediates of armodafinil and related substances. Journal of separation science, 31(6‐7), pp.981-989.
View at Publisher | View at Google Scholar - Pandya, G.P. and Joshi, H.S., (2013). Stability indicating HPTLC method for estimation of modafinil in the bulk and tablet formulation. IOSR J. Pharm. Biol. Sci, 5, pp.22-28.
View at Publisher | View at Google Scholar - Venkata Ramana Reddy, J., Surendra Babu, M.S., Narendra Kumar, M. and Sharma, H.K., (2022). A Direct Standard Headspace Method for the Determination of Chloroacetic Acid and Dichloroacetic Acid in Armodafinil Drug Substance by GC-MS. Analytical Chemistry Letters, 12(1), pp.134-146.
View at Publisher | View at Google Scholar - Wang, W., Xiang, S., Zhou, X., Ji, Y. and Xiang, B., (2011). Enantiomeric separation and determination of the enantiomeric impurity of armodafinil by capillary electrophoresis with sulfobutyl ether-β-cyclodextrin as chiral selector. Molecules, 17(1), pp.303-314.
View at Publisher | View at Google Scholar - AL Azzam, K.M., Saad, B., Adnan, R. and Idiris Saleh, M., (2009). Enantioselective determination of modafinil in pharmaceutical formulations by capillary electrophoresis, and computational calculation of their inclusion complexes. Microchemical Acta, 166(3), pp.311-317.
View at Publisher | View at Google Scholar - Al Azzam, K.M., Saad, B. and Aboul‐Enein, H.Y., (2010). Determination of the binding constants of modafinil enantiomers with sulfated β‐cyclodextrin chiral selector by capillary electrophoresis using three different linear plotting methods. Electrophoresis, 31(17), pp.2957-2963.
View at Publisher | View at Google Scholar - Jonnalagadda, T. and Katakam, S., (2015). A simple visible spectrophotometric method for the determination of armodafinil in bulk and pharmaceutical dosage form. International Journal of Pharmaceutical Sciences and Research, 6(6), p.2579.
View at Publisher | View at Google Scholar

 Clinic
Clinic
