Case Report | DOI: https://doi.org/10.58489/2836-5127/013
Recurrent Primary Spinal Hydatidosis: An Exceptional Etiology of Spinal and Radicular Compression
Radiology Department, Mother-Child Hospital, CHU Mohammed VI, Marrakech Faculty of Medicine of Marrakech, University Cadi Ayyad
*Corresponding Author: M. Mahir, Radiology Department, Mother-Child Hospital, CHU Mohammed VI, Marrakech Faculty of Medicine of Marrakech, University Cadi Ayyad.
Citation: M.Mahir, K.Alloula, B.Zouita, D.Basraoui, H.Jalal (2023), Recurrent primary spinal hydatidosis: an exceptional etiology of spinal and radicular compression, Journal of Radiology Research and Diagnostic Imaging.2(2). DOI: 10.58489/2836-5127/013
Copyright: © 2023 M. Mahir, this is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 06 January 2023 | Accepted: 13 January 2023 | Published: 27 January 2023
Keywords: hydatid cyst; spine; imaging
Abstract
Spinal hydatidosis is an extremely rare occurrence in both children and adults, but it represents the most severe form of bone involvement in echinococcosis. The bone lesions are extensive, with a very progression to neurological complications. Radiologic diagnosis of vertebral hydatidosis, is often difficult. Although surgery is an aggressive treatment therapy for the disease, the recurrent rate remains high. Therefore, it is an imperative management to combine the routine medical imaging with clinical history in the preoperative evaluation and during follow up. We report a case of recurrence of a hydatid cyst with vertebro-medullary localization. The patient underwent 2 operations. We followed up the case for 2 years, thus we discussed our experiences in the diagnosis of spinal hydatidosis.
Introduction
Echinococcosis rarely affects the skeleton. Delays in diagnosisand therapeutic difficulties characterise this disease. To improve the prognosis, early diagnosis with modern imaging is necessary, as well as prevention [1].
Observation
We present the case of a child, aged 8 years, whounderwent surgery in 2020 for a lumbar vertebro- medullary hydatid cyst revealed by a cauda equina syndrome, the hydatid serology was positive and the postoperative pathology examination confirmed the diagnosis. One year later the child was admitted withthe same clinicalsymptoms. LIRM objectified multiple variably sized cysts swelling in paraspinal area of T12-L2 levels and the cyst walls were slightly enhanced. There were neitherclear bound- ariesbetween the cysts and surrounding tissues, nor evidence of spinal cord compression (figure 1) the patient underwent surgery again. Currently, he is consulting for a dorsolumbar spinal syndrome with sphincter disorders. Clinical examination found a 4/5 motor deficit in both lower limbs. A dorsolumbar CTscan confirmed bone lysis of the posteriorwalls of L2, L3 and L4, extended to the left pedicle of L2. In the parenchymal window, the intracanal extension of the multiloculated cystic lesions is evident, giving a grape-like appearance, with walls and partitions discreetly enhanced by PDC. They exert a mass effect on the medullary cord and the nerve roots in front of it (Figure 2, 3). In view of the patient's history, the diagnosis of a recurrence is evoked. The extension workup for other hydatid sites was negative. The patient was operated on. The most complete possible evacuation of the hydatidvesicles, associated with a careful curettage, was performed. Hydatid serologywas positive. Albendazole was prescribed, wAWe present the case of a child, aged 8 years, whounderwent surgery in 2020 for a lumbar vertebro- medullary hydatid cyst revealed by a cauda equina syndrome, the hydatid serology was positive and the postoperative pathology examination confirmed the diagnosis. One year later the child was admitted withthe same clinicalsymptoms. LIRM objectified multiple variably sized cysts swelling in paraspinal area of T12-L2 levels and the cyst walls were slightly enhanced. There were neitherclear bound- ariesbetween the cysts and surrounding tissues, nor evidence of spinal cord compression (figure 1) the patient underwent surgery again. Currently, he is consulting for a dorsolumbar spinal syndrome with sphincter disorders. Clinical examination found a 4/5 motor deficit in both lower limbs. A dorsolumbar CTscan confirmed bone lysis of the posteriorwalls of L2, L3 and L4, extended to the left pedicle of L2. In the parenchymal window, the intracanal extension of the multiloculated cystic lesions is evident, giving a grape-like appearance, with walls and partitions discreetly enhanced by PDC. They exert a mass effect on the medullary cord and the nerve roots in front of it (Figure 2, 3). In view of the patient's history, the diagnosis of a recurrence is evoked. The extension workup for other hydatid sites was negative. The patient was operated on. The most complete possible evacuation of the hydatidvesicles, associated with a careful curettage, was performed. Hydatid serology was positive. Albendazole was prescribed, with clinical and serological monitoring clinical and serological monitoring.
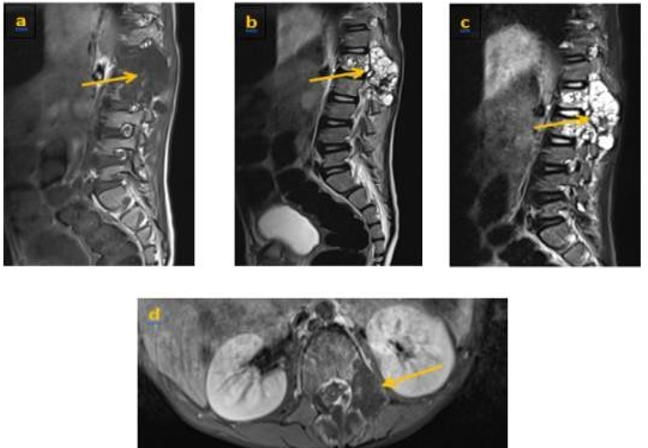
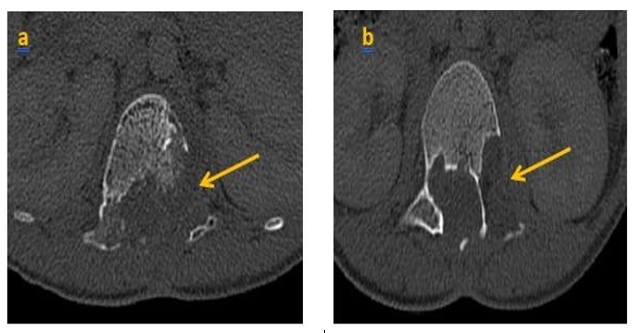
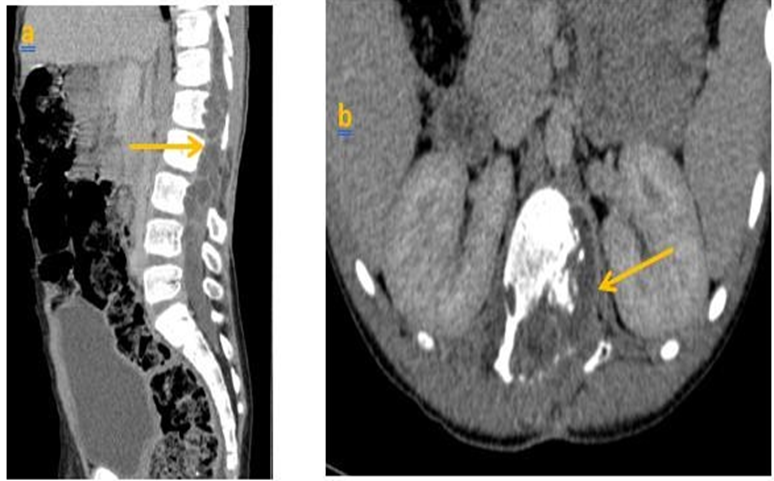
Discussion
Hydatidosis is an anthropozoonosis due to the development of the larval form of Echinococcus granulosus [1]. The diseaseprincipally occurs in dogs, wolves,foxes, sheep, mice, and horses.Humans are only accidental intermediate hosts, and cannottransmit the disease[2]. The infestation starts with ingestion of the eggs of the adult tapeworm.The larvae are released in the duodenum, cross the wall and pass from the portal system to the liver [1]. Notably, hydatidosis infection in humans mainly occurs in the liver and lungs, and the disease has a very long period of asymptomatic incubation until hydatid cysts grow to an extent that results clinical symptoms [2]. Bone involvement is rare: 0.5-2.5% ofall hydatid cases [1]. Spinal involvement involves the vertebral bodies, which are drained by porto-vertebral shunts.The dorsal spine is the most frequently affected (80%). This is followed by the lumbar spine (18%) and the cervical spine (1%) [3]. It is an insidiousdisease, with non-specific clinical signs: pain, ocal soft tissue swelling, pathological fracture [4]. Neurological damage occurs progressively, theclassical presentation is spinal cord compression. Moreover, several retrospective studieshave reported the rate of medullary compression in case of spine hydatidosis ranges between 47% and 73% [2].
Hydatid serology is often negative,becoming positive only at the stage of soft tissue invasion [3, 5].
Diagnosis of hydatidosis not only dependson serological examinations, but also on radiology and biology. Certainly, histopathological diagnosis is thegold standard [2]. The radiological appearance is not specific. Initially, it consists of multiple localised lacunae, which gradually become confluent, creating a grape-like appearance [1]. Costovertebral involvement is suggestive of the diagnosis. Although CT can be used to assess bony involvement, it is not suitable for identification of soft tissue components.Notably, MRI is the pre ferred imaging technique for diagnosisof spinal hydatidosis. They also allow prolonged monitoring. On MRI, hydatid vesicles, in their typicalforms, appear in hyposignal T1 and hypersignal T2 [1]. The recurrent lesion was larger and more significant than previous resultsfrom enhanced MRI. We attributed the difference to bone destruction or disease progression, in addition to theformation of scar tissue after the operation [2]. The differential diagnosismay include tuberculous spondylodiscitis, metastases aAnd aneurysmal cyst. The treatment of spinal hydatidosis is surgical. The role of medical treatment is not negligible for some authors. It seems to improve the painful symptomatology. It has its place especially in inoperable forms and also as an adjuvant therapy to surgical treatment[1]. The prognosisis unpredictable, with a five-yearsurvival rate of approximately 50%, due to the high frequency of recurrence and neurological damage.This poor prognosis is related to the often-incomplete surgical excision [1] and becauseof the absence of anatomical features and the existence of neural structures [2]. In the present case, our patient had undergone 2 previous surgery. The best treatment remains Aprevention of this disease[1].
Conclusion
The spinal location of hydatid cysts is rare and has a poor prognosis. It is characterised by a non-specific clinical symptomatology and a slow but inevitable evolution towards medullaryor radicular compression. The prognosis can be improvedby early diagnosisusing modern imagingtechniques (CT, MRI) and, above all, by the implementation of preventive measures, follow up is also needed to detect and takecare of any potential recurrence.
References
- HOMMADI, A., MOUNACH, J., ZIADI, T., AMHAJI, L., & DRISSI, S. (2008). L'hydatidose vertébro-médullaire: une étiologie exceptionnelle de compression médullaire et radiculaire. La Lettre du rhumatologue, (339), 34-36.
View at Publisher | View at Google Scholar - Xu, X., Cao, J., Wang, J., Wu, T., & Xu, P. (2022). A rare case of recurrent primary dumbbell- shaped spinal hydatidosis. Radiology Case Reports, 17(9), 3224-3227.
View at Publisher | View at Google Scholar - Amira, C., Chaabouni, L., & Zouari, R. (2005). Vertebral hydatidosis: medical imaging and management. A case report. Bulletin de la Societe de Pathologie Exotique (1990), 98(2), 114-117.
View at Publisher | View at Google Scholar - Akhaddar, A., Gourinda, H., El Alami, Z., El Madhi, T., & Miri, A. (1999). Kyste hydatique du sacrum chez une adolescente: A propos d'un cas. Revue du rhumatisme (Ed. française), 66(5), 331-333.
View at Publisher | View at Google Scholar - Karray, S., Zlitni, M., Fowles, J. V., Zouari, O., Slimane, N., Kassab, M. T., & Rosset, P. (1990). Vertebral hydatidosis and paraplegia. The Journal of Bone and Joint Surgery. British volume, 72(1), 84-88.
View at Publisher | View at Google Scholar -
View at Publisher | View at Google Scholar

 Clinic
Clinic
