Research Article | DOI: https://doi.org/0.31579/2835-2971/044
Prevalence of Gestational Diabetes and Pregnancy Outcome of antenatal Patients in Abia State, Nigeria
1Department of Obstetrics and Gynaecology, Abia State University Teaching Hospital, Aba, Nigeria.
2Department of Biochemistry, Lead City University, Ibadan, Oyo State, Nigeria.
*Corresponding Author: Emmanuel M. Akwuruoha, Department of Obstetrics and Gynaecology, Abia State University Teaching Hospital, Aba, Nigeria.
Citation: Emmanuel M. Akwuruoha, Augustine I. Airaodion (2025), Prevalence of Gestational Diabetes and Pregnancy Outcome of antenatal Patients in Abia State, Nigeria, Clinical Pediatrics and Mother Health, 4(1); DOI:10.31579/2835-2971/044
Copyright: © 2025, Emmanuel M. Akwuruoha. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 25 September 2025 | Accepted: 24 October 2025 | Published: 21 November 2025
Keywords: gestational diabetes mellitus; prevalence; pregnancy outcomes; macrosomia; caesarean section; perinatal mortality
Abstract
Background: Gestational diabetes mellitus (GDM) is a significant pregnancy complication associated with adverse maternal and neonatal outcomes. Understanding its prevalence and impact is essential for improving antenatal care strategies in Nigeria.
Objective: To determine the prevalence of GDM and assess its association with pregnancy outcomes among antenatal patients in Abia State University Teaching Hospital (ABSUTH), Aba, Nigeria.
Methods: A hospital-based descriptive cross-sectional study was conducted among 140 pregnant women (gestational age ≥ 24 weeks) attending the ABSUTH antenatal clinic from January to June 2025. Participants were selected using systematic random sampling. Data were collected using an interviewer-administered questionnaire, anthropometric measurements, and laboratory assessments, including fasting plasma glucose and oral glucose tolerance test, following WHO (2013) diagnostic criteria. Pregnancy outcomes were obtained prospectively from antenatal follow-up and delivery records. Data were analyzed using SPSS version 26. Chi-square and correlation tests assessed associations, with p < 0.05 considered significant.
Results: The prevalence of GDM was 10.0% (14/140). Significant positive correlations were observed between GDM and age (r = 0.322, p = 0.001), BMI (r = 0.415, p < 0.001), and fasting plasma glucose (r = 0.542, p < 0.001). Women with GDM had higher rates of macrosomia (42.9% vs 11.1%, p = 0.002), NICU admissions (35.7% vs 7.9%, p = 0.001), caesarean section deliveries (64.3% vs 27.0%, p = 0.004), low Apgar scores at 5 minutes (28.6% vs 5.6%, p = 0.002), and perinatal mortality (14.3% vs 1.6%, p = 0.007) compared to non-GDM participants.
Conclusion: The prevalence of GDM in this study was relatively high, with significant associations with adverse maternal and neonatal outcomes. Routine GDM screening, early diagnosis, and targeted interventions are crucial in reducing pregnancy complications in this population.
Introduction
Gestational diabetes mellitus (GDM), glucose intolerance first recognised during pregnancy, is an increasingly important public-health problem worldwide because of its immediate and long-term consequences for both mothers and offspring [1]. Globally, prevalence estimates vary widely (from under 2% to more than 25%) mainly due to differences in underlying population risk, screening strategies, and the diagnostic criteria applied, but most recent global estimates and syntheses show an upward trend in GDM detection driven by rising maternal age, obesity and improved screening [2,3].
In sub-Saharan Africa (SSA), and specifically in West Africa, GDM is now recognised as a common and under-appreciated contributor to adverse pregnancy outcomes. Recent systematic reviews and meta-analyses focused on SSA report pooled prevalence estimates in the low- to mid-teens (around 12–14%) with substantial between-study heterogeneity; the heterogeneity arises largely from variable screening approaches (universal versus risk-based), different test timing, and multiple diagnostic cutoffs used across studies. This heterogeneity makes direct comparison difficult, but the weight of recent evidence indicates that GDM affects a substantial minority of pregnant women in the region and is not rare [4,5].
Nigeria-specific analyses similarly show wide prevalence ranges across settings — from very low single-site reports to much higher figures in selected high-risk populations — but pooled estimates from national meta-analyses place Nigeria’s pooled prevalence near the low double digits (about 10–11%). The wide spread of reported values (some individual studies reported prevalences under 1% while others exceeded 30–35%) again reflects differences in sampling frames (population-based vs tertiary referral), diagnostic criteria (WHO 1999, WHO 2013/IADPSG, ADA, or two-step approaches), and local risk profiles (urban versus rural populations, maternal BMI and age distributions). These national patterns emphasise both that GDM is common enough to be clinically important in Nigeria and that standardized, facility-level data are needed to guide local policy [6].
GDM is clinically important because of its association with immediate pregnancy and perinatal complications, and with long-term metabolic risk in mother and child. Pregnant women with GDM have an increased risk of hypertensive disorders of pregnancy and primary caesarean delivery; their infants are at higher risk of macrosomia, birth trauma, shoulder dystocia, neonatal hypoglycaemia and respiratory morbidity. Beyond the perinatal period, GDM identifies women at substantially higher risk of later type 2 diabetes and metabolic syndrome, while children exposed in utero to hyperglycaemia have increased risk of obesity and dysglycaemia. In low-resource settings these risks are amplified by gaps in screening, limited specialised diabetes care in pregnancy, and constrained neonatal support, increasing the public-health impact of untreated or poorly managed GDM [3,7].
Despite this recognized burden, facility-level evidence from many parts of Nigeria — and specifically from tertiary centres in some states — remains fragmented or outdated. Few studies provide a recent, hospital-based profile combining unbiased prevalence estimates with systematically collected maternal and perinatal outcome data using contemporary diagnostic criteria. For Abia State University Teaching Hospital (a tertiary referral and teaching centre serving a large and socio-demographically diverse catchment), locally generated data on GDM prevalence, associated maternal risk factors and pregnancy outcomes are essential to inform clinical practice (screening and referral pathways), resource allocation (diabetes in pregnancy clinics, dietetic and obstetric support), and health education priorities in the state. When facility-level evidence is missing or inconsistent, policy decisions risk relying on extrapolations from other regions whose population risk profiles and health-system capacities differ, making local research a priority [5,8].
Accordingly, a well-designed hospital-based study at Abia State University Teaching Hospital that (1) applies a clear, reproducible diagnostic algorithm for GDM, (2) estimates prevalence among consecutive antenatal attendees, and (3) prospectively documents obstetric and neonatal outcomes, will fill an important evidence gap. Such data will help clinicians and health managers understand the local burden of GDM, identify modifiable risk factors (for example maternal obesity or advanced maternal age) amenable to antenatal interventions, and target measures (screening policy, dietary counselling, glucose monitoring and appropriate obstetric planning) to reduce complications. In a broader public-health frame, facility data from the teaching hospital can contribute to state and national estimates while offering a pragmatic platform for piloting context-appropriate management pathways and follow-up strategies to reduce the longer-term cardiometabolic consequences for women and their children [3]. This study, therefore, sought to determine the prevalence of GDM and assess its association with pregnancy outcomes among antenatal patients in Abia State University Teaching Hospital (ABSUTH), Aba, Nigeria.
Materials and Methods
Study Design
This study employed a hospital-based descriptive cross-sectional design to determine the prevalence of gestational diabetes mellitus (GDM) and evaluate associated pregnancy outcomes among antenatal patients receiving care at Abia State University Teaching Hospital (ABSUTH), Aba, Abia State, Nigeria.
Study Area
The study was conducted at the Department of Obstetrics and Gynaecology, Abia State University Teaching Hospital (ABSUTH), located in Aba, Abia State, South-East Nigeria. The hospital is a tertiary healthcare facility that serves as a major referral center for obstetric cases from primary and secondary health facilities within and outside the state. The Antenatal Clinic (ANC) runs daily and offers comprehensive maternal health services, including routine screening for metabolic and obstetric disorders.
Study Population
The target population comprised pregnant women attending ANC at ABSUTH during the study period. The participants were in the second or third trimester of pregnancy and met the eligibility criteria.
Inclusion Criteria
- Pregnant women aged 18–45 years.
- Gestational age ≥ 24 weeks confirmed by last menstrual period (LMP) and/or early ultrasound.
- Willingness to participate and provide informed consent.
Exclusion Criteria
- Known cases of pre-gestational diabetes mellitus (Type 1 or Type 2).
- Multiple gestations.
- Presence of severe medical conditions (e.g., chronic renal failure, severe anemia) that could affect glucose metabolism.
- Declined consent.
Sample Size Determination
The sample size was determined using Cochran's formula for estimating population proportions, as outlined by Ezebuiro et al. [9]:
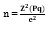
The formula components are defined as follows:
- n represents the minimum required sample size.
- Z is set at 1.96, corresponding to a 95% confidence level.
- P denotes the established prevalence of contraceptive use among women in Nigeria.
- e signifies the allowable margin of error, fixed at 5% (0.05).
q = 1 - p
A recent study conducted by Ajiboye et al. [10] reported the prevalence of GDM in Nigeria as 9%
P = 9% = 0.09
q = 1 – 0.09
= 0.91
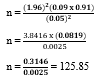
The minimum sample size was 126, but it was adjusted to 140 to account for a 10% non-response rate.
Sampling Technique
A systematic random sampling technique was used. Using the ANC attendance register, the sampling interval was determined by dividing the estimated number of eligible pregnant women attending ANC during the study period by the required sample size [11]. The first participant was selected randomly, and every 5th eligible woman was subsequently recruited until the sample size was attained.
Study Duration
The study was conducted over a 6-month period from January to June 2025.
Data Collection Instruments
Data were collected using a structured interviewer-administered questionnaire adapted from previously validated tools for GDM screening studies. The questionnaire consisted of the following sections:
- Sociodemographic data – age, marital status, educational level, occupation.
- Obstetric history – gravidity, parity, history of macrosomia, stillbirth, and preeclampsia.
- Family and medical history – family history of diabetes, hypertension.
The following parameters were determined:
Anthropometric measurements – weight, height, and body mass index (BMI).
Clinical and laboratory results – blood pressure, random blood glucose (RBG), fasting plasma glucose (FPG), oral glucose tolerance test (OGTT).
Pregnancy outcomes – mode of delivery, birth weight, Apgar score, neonatal complications, and maternal complications.
Screening for Gestational Diabetes Mellitus
Screening and diagnosis of GDM followed the World Health Organization diagnostic criteria [12].
- Step 1 – Initial Screening: All consenting participants underwent fasting plasma glucose (FPG) testing after an overnight fast of 8–10 hours.
- Step 2 – OGTT: Participants with FPG ≥ 5.1 mmol/L and ≤ 6.9 mmol/L, or those at high risk, underwent a 75 g oral glucose tolerance test. Blood samples were collected at baseline (fasting), 1 hour, and 2 hours post-glucose load.
- Diagnostic thresholds (WHO, 2013):
- FPG ≥ 5.1 mmol/L (92 mg/dL)
- 1-hour post-load ≥ 10.0 mmol/L (180 mg/dL)
- 2-hour post-load ≥ 8.5 mmol/L (153 mg/dL).
Diagnosis of GDM was made if any of the above thresholds were met.
Assessment of Pregnancy Outcomes
Pregnancy outcome data were collected prospectively from antenatal follow-up records and delivery registers. The maternal outcomes assessed included:
- Mode of delivery (spontaneous vaginal delivery, assisted vaginal delivery, elective/emergency caesarean section).
- Hypertensive disorders in pregnancy (gestational hypertension, preeclampsia, eclampsia).
- Obstetric complications (postpartum hemorrhage, obstructed labour).
Fetal and neonatal outcomes assessed included:
- Birth weight categories (low birth weight < 2>
- Apgar scores at 1 and 5 minutes.
- Neonatal intensive care unit (NICU) admission.
- Perinatal mortality.
Data Collection Procedure
Trained research assistants (midwives and resident doctors) administered the questionnaires, conducted anthropometric measurements using standardized protocols, and collected venous blood samples under aseptic conditions. Laboratory analyses were performed in the ABSUTH Chemical Pathology Department by certified laboratory scientists.
Quality Control
The questionnaire was pretested on 20 antenatal patients at Rhema University Teaching Hospital, Aba to ensure clarity and reliability. All measuring instruments (weighing scales, glucometers) were calibrated daily. Blood glucose measurements adhered strictly to WHO-recommended laboratory protocols. Supervisors conducted daily checks of completed questionnaires for completeness and accuracy.
Ethical Considerations
This study was approved by the Abia State University Teaching Hospital Research Ethics Committee with reference number ABSUTH/MAC/117//VOL.II/78. Written informed consent was obtained from all participants after explaining the purpose, procedures, potential risks, and benefits of the study. Confidentiality of participants’ data was strictly maintained through coded identifiers and restricted access to data files.
Data Analysis
Data were entered and analyzed using Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics (frequencies, percentages, means, and standard deviations) were computed for sociodemographic and clinical characteristics. The prevalence of GDM was calculated as the proportion of participants diagnosed with GDM out of the total sample. Associations between GDM status and pregnancy outcomes were examined using the Chi-square test for categorical variables, and independent-samples t-test for continuous variables. A p-value < 0>
Results
The study involved 140 pregnant women, with the majority aged between 30–34 years (26.43%), followed by 35–39 years (22.14%) (Table 1). Most participants were married (90.71%), had attained tertiary education (43.57%), and were traders (33.57%). Obstetric history revealed that 44.29% had 3–4 previous pregnancies, 59.29% were multiparous, and 13.57% reported a history of macrosomia (Table 2). Family histories of hypertension (29.29%) and diabetes mellitus (18.57%) were also noted.
The prevalence of gestational diabetes mellitus (GDM) was 10.00% (Figure 1). Mean clinical and anthropometric measurements included age 31.8 ± 5.9 years, BMI 29.3 ± 4.8 kg/m², systolic BP 121.6 ± 12.8 mmHg, and fasting plasma glucose 4.9 ± 0.8 mmol/L (Table 3).
Pregnancy outcomes (Table 4) showed spontaneous vaginal delivery as the most common mode (63.57%), while 30.71% underwent caesarean section. Birth weight ranged mostly between 2.5–3.9 kg (73.57%), with macrosomia observed in 14.29% of newborns. NICU admission occurred in 10.71% of cases, and perinatal mortality was 2.86%.
Awareness of GDM risk factors was high (Table 5), with over 85% of respondents agreeing that excess weight, family history of diabetes, and poor lifestyle habits increase GDM risk. Most participants strongly agreed that screening for GDM during pregnancy is important (50.71%) and that healthy diet and exercise can reduce risk (52.14% agreement).
Correlation analysis (Table 6) indicated significant positive associations between GDM and age (r = 0.322, p = 0.001), BMI (r = 0.415, p < 0.001), systolic BP (r = 0.276, p = 0.002), diastolic BP (r = 0.219, p = 0.011), fasting plasma glucose (r = 0.542, p < 0.001), and birth weight (r = 0.238, p = 0.007). Chi-square analysis (Table 7) showed that GDM-positive women had significantly higher rates of macrosomia (42.86% vs 11.11%, p = 0.002), NICU admission (35.71% vs 7.94%, p = 0.001), caesarean section (64.29% vs 26.98%, p = 0.004), low Apgar scores (28.57% vs 5.56%, p = 0.002), and perinatal mortality (14.29% vs 1.59%, p = 0.007).
| Variable | Frequency (n) | Percentage (%) |
| Age group (years) | ||
| 18–24 | 22 | 15.71 |
| 25–29 | 28 | 20.00 |
| 30–34 | 37 | 26.43 |
| 35–39 | 31 | 22.14 |
| 40–45 | 22 | 15.71 |
| Marital status | ||
| Married | 127 | 90.71 |
| Single | 9 | 6.43 |
| Divorced/Separated | 4 | 2.86 |
| Educational level | ||
| No formal education | 8 | 5.71 |
| Primary | 19 | 13.57 |
| Secondary | 52 | 37.14 |
| Tertiary | 61 | 43.57 |
| Occupation | ||
| Unemployed | 23 | 16.43 |
| Trader | 47 | 33.57 |
| Civil servant | 39 | 27.86 |
| Artisan | 18 | 12.86 |
| Professional | 13 | 9.29 |
Table 1: Sociodemographic Characteristics of Participants (n = 140)
| Variable | Frequency (n) | Percentage (%) |
| Gravidity | ||
| 1–2 | 54 | 38.57 |
| 3–4 | 62 | 44.29 |
| ≥5 | 24 | 17.14 |
| Parity | ||
| Nulliparous | 37 | 26.43 |
| Multiparous (1–4) | 83 | 59.29 |
| Grand multiparous (≥5) | 20 | 14.29 |
| History of macrosomia | 19 | 13.57 |
| History of stillbirth | 12 | 8.57 |
| History of preeclampsia | 15 | 10.71 |
| Family history of DM | 26 | 18.57 |
| Family history of HTN | 41 | 29.29 |
Table 2: Obstetric and Medical History of Participants
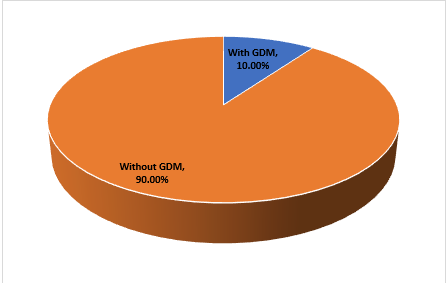
Figure 1: Prevalence of Gestational Diabetes Mellitus
| Parameter | Mean ± SD |
| Age (years) | 31.8 ± 5.9 |
| Weight (kg) | 78.6 ± 11.4 |
| Height (m) | 1.64 ± 0.07 |
| BMI (kg/m²) | 29.3 ± 4.8 |
| Systolic BP (mmHg) | 121.6 ± 12.8 |
| Diastolic BP (mmHg) | 78.9 ± 9.4 |
| Fasting plasma glucose (mmol/L) | 4.9 ± 0.8 |
| 1-hour OGTT (mmol/L) | 8.6 ± 1.5 |
| 2-hour OGTT (mmol/L) | 6.9 ± 1.3 |
Table 3: Clinical and Anthropometric Measurements
| Variable | Frequency (n) | Percentage (%) |
| Mode of delivery | ||
| Spontaneous vaginal | 89 | 63.57 |
| Assisted vaginal | 8 | 5.71 |
| Elective C-section | 21 | 15.00 |
| Emergency C-section | 22 | 15.71 |
| Birth weight | ||
| < 2> | 17 | 12.14 |
| 2.5–3.9 kg | 103 | 73.57 |
| ≥ 4.0 kg (Macrosomia) | 20 | 14.29 |
| Apgar score at 5 min <7> | 11 | 7.86 |
| NICU admission | 15 | 10.71 |
| Perinatal mortality | 4 | 2.86 |
Table 4: Pregnancy Outcomes
| Statement | Strongly Disagree (%) | Disagree (%) | Neutral (%) | Agree (%) | Strongly Agree (%) |
| Excess weight increases risk of GDM | 2 (1.43) | 6 (4.29) | 11 (7.86) | 72 (51.43) | 49 (35.00) |
| Family history of diabetes is a major risk factor | 3 (2.14) | 8 (5.71) | 19 (13.57) | 66 (47.14) | 44 (31.43) |
| GDM can lead to complications for the baby | 1 (0.71) | 5 (3.57) | 13 (9.29) | 69 (49.29) | 52 (37.14) |
| Screening for GDM is important during pregnancy | 0 (0.00) | 4 (2.86) | 7 (5.00) | 58 (41.43) | 71 (50.71) |
| Healthy diet and exercise can reduce risk of GDM | 1 (0.71) | 3 (2.14) | 14 (10.00) | 73 (52.14) | 49 (35.00) |
Table 5: Awareness of Risk Factors for GDM
| Variable | r-value | p-value |
| Age (years) | 0.322 | 0.001* |
| BMI (kg/m²) | 0.415 | <0> |
| Systolic BP (mmHg) | 0.276 | 0.002* |
| Diastolic BP (mmHg) | 0.219 | 0.011* |
| Fasting plasma glucose | 0.542 | <0> |
| Birth weight | 0.238 | 0.007* |
Table 6: Correlation Analysis Between GDM and Continuous Variables
*Significant at p < 0>
| Outcome | GDM Positive (n=14) | GDM Negative (n=126) | χ² value | p-value |
| Macrosomia | 6 (42.86%) | 14 (11.11%) | 9.874 | 0.002* |
| NICU admission | 5 (35.71%) | 10 (7.94%) | 10.982 | 0.001* |
| C-section (any) | 9 (64.29%) | 34 (26.98%) | 8.142 | 0.004* |
| Low Apgar (<7> | 4 (28.57%) | 7 (5.56%) | 9.871 | 0.002* |
| Perinatal mortality | 2 (14.29%) | 2 (1.59%) | 7.356 | 0.007* |
Table 7: Chi-Square Analysis Between GDM Status and Pregnancy Outcomes
*Significant at p < 0>
Discussion
The present study found a gestational diabetes mellitus (GDM) prevalence of 10.0% among 140 antenatal patients in Abia State, Nigeria. This estimate is broadly consistent with recent pooled and local reports from Nigeria and sub‑Saharan Africa, which place GDM prevalence in the single digits to low teens depending on diagnostic criteria and population screened. A recent systematic review and meta‑analysis specifically focusing on Nigeria reported a pooled prevalence around 11%, showing substantial heterogeneity across studies but overall confirming that GDM is a common problem in the country [6]. A more recent study carried out in Ilorin, Nigeria reported 9% prevalence of GDM. Our observed 10% therefore aligns well with these national averages and suggests that Abia State experiences a GDM burden comparable to other parts of Nigeria and the region.
Age and adiposity emerged as important correlates in our data: maternal age (r = 0.322, p = 0.001) and BMI (r = 0.415, p < 0 xss=removed>
The downstream pregnancy outcomes observed in our cohort indicate clinically important consequences of GDM. Women with GDM in our sample had higher proportions of macrosomia (42.9% vs 11.1%; χ² = 9.874, p = 0.002), NICU admissions (35.7% vs 7.9%; χ² = 10.982, p = 0.001) and cesarean delivery (64.3% vs 27.0%; χ² = 8.142, p = 0.004) compared with women without GDM. These associations are consistent with meta‑analytic and primary‑study evidence from both Africa and high‑income settings showing that GDM substantially increases the risk of large‑for‑gestational‑age infants, operative delivery, neonatal morbidity requiring intensive care, and other perinatal complications. A pooled analysis of sub‑Saharan data reported elevated risks for fetal macrosomia and cesarean section among mothers with GDM, and tertiary‑centre Nigerian studies have similarly linked maternal hyperglycaemia to higher rates of macrosomia and associated delivery complications [5,14]. The biological plausibility is clear: maternal hyperglycaemia exposure during late gestation stimulates fetal insulin production and somatic growth, predisposing to macrosomia and birth complications that increase the likelihood of operative delivery and neonatal support.
Perinatal mortality in our cohort was low in absolute terms (2.86% overall) but disproportionately concentrated among GDM cases (14.3% of the GDM group vs 1.6% without GDM; χ² = 7.356, p = 0.007). While many large international cohorts do not show dramatically higher neonatal mortality for infants of mothers with well‑managed GDM, studies in resource‑limited settings frequently report worsened perinatal outcomes when detection or glycaemic control is suboptimal. Our finding therefore reinforces the concern that where screening, glycaemic control, intrapartum management, and neonatal monitoring are not uniformly robust, GDM can contribute to excess perinatal mortality. This observation is in line with region‑specific reports linking maternal hyperglycaemia with an increased risk of stillbirth, neonatal hypoglycaemia and early neonatal death when antenatal and peripartum care are inconsistent [15,16].
The anthropometric and clinical profile of our cohort—mean BMI 29.3 ± 4.8 kg/m² and mean age 31.8 ± 5.9 years—underscores why GDM is common here. Increasing rates of maternal overweight and obesity in many Nigerian cities and urbanizing regions have been documented recently, and these shifts in maternal body composition are hypothesized to be a key driver of rising GDM prevalence. Population studies from Nigeria report growing maternal overweight and obesity burdens that are associated with adverse pregnancy outcomes, including GDM and macrosomia; our data add local confirmation that excess maternal weight is a central modifiable determinant [17,18].
Maternal awareness in this study was relatively high for several important risk and prevention concepts: over 85% of participants agreed or strongly agreed that excess weight increases GDM risk, and more than 90% agreed that screening is important and that healthy diet and exercise can reduce risk (Table 5). This level of awareness compares favourably with some Nigerian hospital‑based surveys that have reported variable but often limited knowledge about GDM; other institutional studies show important gaps in awareness and misconceptions among pregnant women, particularly in lower‑education or rural subgroups. The relatively high awareness in our sample—where 43.6% had tertiary education—suggests that knowledge alone is not sufficient to prevent GDM unless it is accompanied by effective public‑health support, preconception counselling, weight management programs, and structured antenatal screening and treatment pathways [19].
From a public‑health standpoint, our findings carry clear implications. First, the observed prevalence (10%) and the demonstrated adverse outcomes (macrosomia, increased cesarean rate, NICU admissions, and elevated perinatal mortality among GDM cases) argue for consistent, universal screening strategies adapted to local resources and accompanied by documented care pathways. The literature supports that early identification and active management (dietary counselling, glucose monitoring, medical therapy when indicated, and planned intrapartum monitoring) reduce some neonatal complications and help guide mode‑of‑delivery decisions. Second, the strong correlation with BMI points to the value of upstream interventions: strengthening preconception and antenatal programs that address maternal nutrition, physical activity and weight management could help reduce GDM incidence. Third, improved integration of newborn monitoring protocols (for hypoglycaemia and respiratory adaptation), timely neonatal support, and clear referral pathways for high‑risk births should be priorities given the elevated NICU admissions observed among GDM mothers [5,20].
Conclusions
The 10% prevalence of GDM observed among antenatal patients in Abia State, Nigeria, together with the clear associations with maternal age and BMI and the elevated risks of macrosomia, operative delivery, NICU admission, and perinatal death, underscore that GDM is a clinically and public‑health relevant problem in this setting. These results corroborate recent Nigerian and sub‑Saharan evidence that maternal overweight and family history are key drivers, and that GDM contributes to adverse perinatal outcomes when not optimally detected and managed. We recommend strengthening routine, standardized screening for GDM in antenatal services; implementing focused interventions to reduce maternal overweight before and during pregnancy; ensuring clear, evidence‑based treatment algorithms; and improving neonatal surveillance and care pathways for infants of mothers with GDM. Future research should examine the effectiveness of context‑specific screening strategies, long‑term maternal metabolic follow‑up after pregnancy, and the cost‑effectiveness of integrated programs to reduce both GDM incidence and its downstream complications.
References
- Plows, J. F., Stanley, J. L., Baker, P. N., Reynolds, C. M., & Vickers, M. H. (2018). The Pathophysiology of Gestational Diabetes Mellitus. International journal of molecular sciences, 19(11):3342.
View at Publisher | View at Google Scholar - Wang, H., Li, N., Chivese, T., Werfalli, M., Sun, H., Yuen, L., Hoegfeldt, C. A., Elise Powe, C., Immanuel, J., Karuranga, S., Divakar, H., Levitt, N., Li, C., Simmons, D., Yang, X., & IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group (2022). IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes research and clinical practice, 183:109050.
View at Publisher | View at Google Scholar - Basil, B., Mba, I. N., Gav, T. A., Myke-Mbata, B. K., Swende, T. Z., & Adebisi, S. A. (2023). Rising prevalence of gestational diabetes mellitus and its associated risk factors in Makurdi, North-Central Region of Nigeria. African health sciences, 23(4):348-355.
View at Publisher | View at Google Scholar - Abera, D. A., Larbie, C., Abugri, J., Ofosu, M., Mutocheluh, M., & Dongsogo, J. (2024). Prevalence and Predictors of Gestational Diabetes Mellitus in Sub-Saharan Africa: A 10-Year Systematic Review. Endocrinology, diabetes & metabolism, 7(3):00478.
View at Publisher | View at Google Scholar - Chileshe, M., Imanga, F., Zulu, A.C. and Kazonga, E. (2025) Prevalence, Predictors, and Outcomes of Gestational Diabetes Mellitus in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Open Journal of Epidemiology, 15: 421-438.
View at Publisher | View at Google Scholar - Azeez, T. A., Abo-Briggs, T., & Adeyanju, A. S. (2021). A systematic review and meta-analysis of the prevalence and determinants of gestational diabetes mellitus in Nigeria. Indian Journal of Endocrinology and Metabolism, 25(3):182-190.
View at Publisher | View at Google Scholar - Druye, A. A., Owusu, G., Yeboa, N. K., Boso, C. M., Berchie, G. O., Nabe, B., Abraham, S. A., Nsatimba, F., Agyare, D. F., Agyeiwaa, J., Opoku-Danso, R., Okantey, C., Ofori, G. O., Kagbo, J. E., Obeng, P., Amoadu, M., & Azu, T. D. (2024). Self-management interventions for gestational diabetes in Africa: A scoping review. BMC Pregnancy and Childbirth, 24:549.
View at Publisher | View at Google Scholar - Mwanri, A. W., Kinabo, J., Ramaiya, K., & Feskens, E. J. M. (2015). Gestational diabetes mellitus in sub‐Saharan Africa: Systematic review and metaregression on prevalence and risk factors. Tropical Medicine & International Health, 20(8): 983–1002.
View at Publisher | View at Google Scholar - Ezebuiro, E. I., Adesina, O. O., Alumona, F. C., Abali, I. O., Ezirim, E. O., Akwuruoha, E. M., Mba, K. K., Mba, C. J., Onyemereze, C. O., & Airaodion, A. I. (2024). Awareness and acceptance of obstetric epidural analgesia among expectant mothers in Southeast Nigeria. International Journal of Reproductive Research, 3(2).
View at Publisher | View at Google Scholar - Ajiboye, A. D., Adesina, K. T., Abdul, I. F., Ezeoke, G. G., Biliaminu, A. S., Fehintola, A. O., & Ayegbusi, E. O. (2024). Prevalence of Gestational Diabetes and Pregnancy Outcome of antenatal patients in Ilorin. Nigerian Medical Journal: Journal of the Nigeria Medical Association, 64(6), 780.
View at Publisher | View at Google Scholar - Akwuruoha, E. M., Onwube, O. C., Akwuruoha, C. U., & Airaodion, A. I. (2025). Prevalence, causes and psychological effects of miscarriage among women in Abia State University Teaching Hospital, Aba, Nigeria. American Journal of Biomedical Science & Research, 27(5), 850-856.
View at Publisher | View at Google Scholar - World Health Organization. (2013). Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy. https://iris.who.int/bitstream/handle/10665/85975/WHO_NMH_MND_13.2_eng.pdf
View at Publisher | View at Google Scholar - Ogu, R., Maduka, O., Agala, V., Obuah, P., Horsfall, F., Azi, E., Nwibubasa, C., Edewor, U., Porbeni, I., John, O., Orazulike, N., Kalio, D., Okagua, K., Edet, C., Harry, A., Ugboma, H., & Abam, C. (2022). The Case for Early and Universal Screening for Gestational Diabetes Mellitus: Findings from 9314 Pregnant Women in a Major City in Nigeria. Diabetes therapy: research, treatment and education of diabetes and related disorders, 13(10):1769-1778.
View at Publisher | View at Google Scholar - Olokor, O. E., Onakewhor, J. U., & Aderoba, A. K. (2015). Determinants and outcome of fetal macrosomia in a Nigerian tertiary hospital. Nigerian medical journal: journal of the Nigeria Medical Association, 56(6):411-415.
View at Publisher | View at Google Scholar - Battarbee, A. N., Venkatesh, K. K., Aliaga, S., & Boggess, K. A. (2020). The association of pregestational and gestational diabetes with severe neonatal morbidity and mortality. Journal of Perinatology, 40(2):232-239.
View at Publisher | View at Google Scholar - Abudu, O. O., & Awonuga, A. O. (1989). Fetal macrosomia and pregnancy outcome in Lagos. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics, 28(3): 257-262.
View at Publisher | View at Google Scholar - Adeoye, I.A., Bamgboye, E.A. & Omigbodun, A.O. Maternal obesity, lifestyle factors and associated pregnancy outcomes in Ibadan, Nigeria: a Nigerian cohort study. Sci Rep 15:11129 (2025).
View at Publisher | View at Google Scholar - Rahnemaei, F. A., Abdi, F., Kazemian, E., Shaterian, N., Shaterian, N., & Behesht Aeen, F. (2022). Association between body mass index in the first half of pregnancy and gestational diabetes: A systematic review. SAGE open medicine, 10, 20503121221109911.
View at Publisher | View at Google Scholar - Chikeme, P. C. (2024). Gestational diabetes mellitus: Awareness, risk factors, perceived effects, and lifestyle intervention among pregnant women in a Nigerian tertiary health institution. Indian Journal of Medical Sciences, 76: 22-27.
View at Publisher | View at Google Scholar - Hillick, D., O’Reilly, D., Murphy, L., Breathnach, F., & McCallion, N. (2025). Increased risk of admission to neonatal intensive care unit in neonates born to mothers with pregestational diabetes. European Journal of Pediatrics, 184:354.
View at Publisher | View at Google Scholar

 Clinic
Clinic
