Research Article | DOI: https://doi.org/DOI:10.31579/2835-835X/039
Pharmacophore Modeling and 3D QSAR Analysis of Pyrazole-3-Carbohydrazone Derivatives as Dipeptidyl Peptidase IV Inhibitors for Type II Anti-Diabetic Therapy
1 IPL Research, Aishbagh. Lucknow, India.
2 Amity University Uttar Pradesh, Lucknow.
*Corresponding Author: Krishna Sarma Pathy, IPL Research, Aishbagh. Lucknow, India.
Citation: Krishna S. Pathy, Rachana Chaturvedi, Pathy S. Sarma, (2023), Pharmacophore Modeling and 3D QSAR Analysis of Pyrazole-3-Carbohydrazone Derivatives as Dipeptidyl Peptidase IV Inhibitors for Type II Anti-Diabetic Therapy, Clinical Trials and Case Studies, 2(5); DOI:10.31579/2835-835X/039
Copyright: © 2023, Krishna Sarma Pathy. This is an open-access artic le distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 29 August 2023 | Accepted: 20 September 2023 | Published: 09 October 2023
Keywords: motor car accidents; genito urinary trauma; fracture penis; bullet injury; iatrogenic trauma
Abstract
This study delves into the design of promising Type II anti-diabetic agents acting as inhibitors of Dipeptidyl Peptidase-IV (DPP-IV). Given the significance of Type 2 Diabetes Mellitus (T2DM) as a prevalent metabolic disorder, the pursuit of improved therapies is essential. Leveraging 3D QSAR and pharmacophore modeling techniques, this research identifies critical structural elements pivotal to the biological efficacy of cyanopyrrolidine derivatives. The objective is to provide invaluable insights fostering the development of potent Type II anti-diabetic agents.
Introduction
Type 2 Diabetes Mellitus (T2DM) is a widely recognized chronic metabolic ailment associated with heightened morbidity and mortality. Notable trials such as the Diabetes Control and Complications Trial, the Stockholm Diabetes Intervention Study, and the United Kingdom Prospective Diabetes Study have substantiated the advantages of enhanced glucose control in reducing complications. Underlying T2DM are three core anomalies: insulin resistance, diminished insulin secretion, and excessive hepatic glucose production. Current therapeutic options encounter limitations encompassing safety concerns, efficacy sustainability, and dosing inconveniences. Adverse effects commonly linked to existing agents encompass hypoglycemia, weight gain, and gastrointestinal intolerance. Dipeptidyl peptidase-4 (DPP-4) inhibitors, exemplified by saxagliptin, offer distinctive mechanisms with potential for improved safety, tolerability, and effectiveness. Approved agents such as sitagliptin (Januvia®) and vildagliptin (Galvus®) exemplify this class.
Methods
This article presents a comprehensive exploration involving 3D QSAR and pharmacophore modeling applied to substituted cyanopyrrolidines as potential Type II anti-diabetic agents and DPP-IV inhibitors. Cyanopyrrolidines, a chemically significant class, have shown diverse medical relevance. Various researchers have reported the anti-diabetic potential of cyanopyrrolidine derivatives. The utilization of 3D QSAR aims to unravel the intricate three-dimensional structural attributes pivotal for their anti-diabetic activity. The obtained 3D QSAR model (characterized by a squared correlation coefficient, r2, of 0.9945 and a cross-validated squared correlation coefficient, q2, of 0.9866) attests to its statistical significance and predictive proficiency. The insights derived from this model shed light on the structural motifs driving the inhibitory potency of cyanopyrrolidines. Additionally, pharmacophore modeling has been employed to discern the structural prerequisites crucial for the biological efficacy of these compounds.
This study underscores the critical role of pharmacophore modeling and 3D QSAR analysis in elucidating the intricate structural attributes that underpin the efficacy of substituted cyanopyrrolidines as Type II anti-diabetic agents and DPP-IV inhibitors. The outcomes have potential implications for advancing the development of potent therapies in the realm of Type II diabetes treatment
Type II Diabetes Mellitus (T2DM) is a persistent metabolic ailment characterized by three primary anomalies: insulin resistance, diminished insulin secretion, and excessive hepatic glucose production. However, existing treatments exhibit limitations in terms of safety, effectiveness, and tolerability. To address this, there is potential in Dipeptidyl Peptidase-IV (DPP-IV) inhibitors like saxagliptin, which operate through distinct mechanisms. This study delves into the creation, correlation of structure and activity, and modeling of pharmacophores for cyanopyrrolidine derivatives, aiming to establish them as potential DPP-IV inhibitors.
Saxagliptin belongs to the category of oral antidiabetic agents referred to as DPP-IV inhibitors or "incretin enhancers." The phase III trial initiative for saxagliptin encompassed investigations involving both standalone administration and concurrent use with other established antidiabetic medications such as metformin, sulphonylureas, and thiazolidinediones. Among the evolving classes of antidiabetic drugs for type 2 diabetes, DPP-IV inhibitors, including vildagliptin (Galvus®) and sitagliptin (Januvia®), are already endorsed and employed clinically. The appeal of these agents lies in their ability to sustainably lower HbA1c levels—a pivotal marker of blood glucose management—via an orally administered, well-tolerated approach, distinguishing them from many conventional oral antidiabetic drug The invention primarily revolves around DPP4 inhibitors, particularly in the context of a novel formulation involving cyano-pyrrolidine-based compounds. Saxagliptin, represented as (1S,3S,5S)-2-((2S)-2-amino-2-(3-hydroxyadamantan-1-yl) acetyl)-2-azabicyclo[3.1.0]hexane-3-carbonitrile, falls within the scope of cyano-pyrrolidine-based DPP4 inhibitors. Its chemical structure is as follows: [Chemical formula representation].
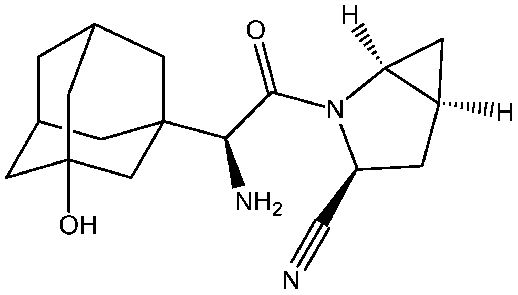
Saxagliptin, in the form of its hydrochloride salt, is marketed under the trade name ONGLYZA® by Bristol-Myers Squibb for the treatment of type 2 diabetes mellitus. Each film-coated tablet of ONGLYZA for oral use contains either 2.79 mg saxagliptin hydrochloride (anhydrous) equivalent to 2.5 mg saxagliptin, or 5.58 mg saxagliptin hydrochloride (anhydrous) equivalent to 5 mg saxagliptin and the following inactive ingredients: lactose monohydrate, microcrystalline cellulose, croscarmellose sodium, and magnesium stearate. In addition, the film coating contains the following inactive ingredients: polyvinyl alcohol, polyethylene glycol, titanium dioxide, talc, and iron oxides.
Thermodynamic Degradation of Saxagliptin,
cyclic amidine ("AMD") and oxamidine ("OXAMD") respectively. The hydrolysis of amidine to diketopiperazine occurs in the presence of water.
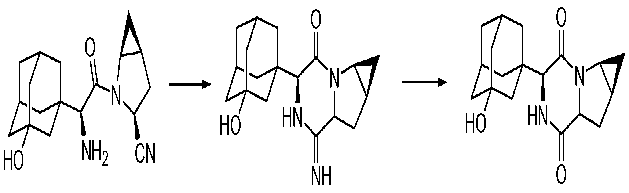
The intramolecular cyclization reaction leading to the formation of a cyclic amidine can occur in both the solid state and the solution state. Furthermore, this reaction can be exacerbated by utilizing processing conditions like wet granulation, roller compaction, or tabletting.
This chemical instability necessitates the provision of conditions and excipients that either minimize or prevent this undesired reaction during the manufacturing of saxagliptin formulations.
The solid-state structures of (lS,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxy-l-adamantyl) acetyl]-2-azabicyclo [3.1.0] hexane-3-carbonitrile, referred to as Saxagliptin, can exist in the form of stable amorphous and crystalline solids. Crystalline solid exhibits long-range order, while amorphous solids lack this order, resembling a frozen liquid with solid-like rheological properties.
When a compound like saxagliptin is transformed into an amorphous state but not fully dispersed within a polymer matrix, leading to amorphous clusters embedded in the polymer, it is termed a "glass suspension." This results in a glass suspension with two distinct glass transition temperatures, arising from the amorphous API and the polymer.
Discussion
The discussion section delves into the methodology, results, and implications of the study. It outlines the synthesis of saxagliptin, a DPP-IV inhibitor, using 3D QSAR and pharmacophore modeling to uncover structural features crucial for Type II anti-diabetic activity. A predictive model was created using a training set of molecules, with statistical analysis evaluating its forecasting accuracy. The study highlights molecular attributes impacting the inhibitory potency of cyanopyrrolidine derivatives, as identified through pharmacophore modeling for interaction with the DPP-IV receptor.
Amorphous solids generally exhibit greater solubility compared to crystalline forms due to their lack of long-range order and higher surface area. To enhance the solubility of a crystalline solid, transforming the active pharmaceutical ingredient into an amorphous form is advantageous.
When a crystalline material is heated to its melting point (Tm), it transitions from a solid to a liquid state, with reversible behavior upon cooling. Rapid cooling below Tm can prevent crystallization, resulting in a supercooled liquid. If this supercooled liquid is further cooled to its glass transition temperature (Tg), molecules kinetically solidify, forming a glass. While molecules in a supercooled liquid have higher mobility than in a glassy state, the latter still exhibits some mobility.
Due to this mobility, it is beneficial for the glass transition temperature of the active pharmaceutical ingredient to be significantly higher (e.g., at least 20°C, preferably 30°C, or even 40°C) than the actual storage conditions.
Amorphous Saxagliptin, with a relatively low Tg of about 54°C, tends to recrystallize under storage conditions. Stabilizing the amorphous form by increasing its Tg is crucial to prevent recrystallization. This can be achieved by mixing the API with a second component, typically polymers that decrease the mobility of Saxagliptin molecules and thwart recrystallization.
Two approaches can be used to prepare glass solutions via the spray drying technique: using Saxagliptin as a salt or as a free base in situ with an acid. This yields Saxagliptin dispersed within a polymer-formed matrix.
The intramolecular cyclization process that leads to the formation of a cyclic amidine can take place in both the solid and solution states. Moreover, this reaction can be intensified by employing various processing conditions such as wet granulation, roller compaction, or tabletting.
This chemical instability necessitates the establishment of conditions and additives that can either reduce or prevent this undesirable reaction during the manufacturing of saxagliptin formulations.
The solid-state structures of Saxagliptin, specifically (lS,3S,5S)-2-[(2S)-2-amino-2-(3-hydroxy-l-adamantyl) acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile, can exist in the form of both stable amorphous and crystalline solids. Crystalline solids exhibit a well-ordered structure over long distances, whereas amorphous solids lack this order, resembling a frozen liquid with solid-like rheological properties.
When a compound like saxagliptin transitions into an amorphous state but isn't fully dispersed within a polymer matrix, resulting in amorphous clusters embedded in the polymer, it is referred to as a "glass suspension." This leads to a glass suspension that possesses two distinct glass transition temperatures, stemming from the amorphous active pharmaceutical ingredient (API) and the polymer.
The discussion section delves into the study's methodology, outcomes, and implications. It outlines the synthesis of saxagliptin, a DPP-IV inhibitor, using 3D QSAR and pharmacophore modeling to identify critical structural features for Type II anti-diabetic activity. A predictive model was developed using a training set of molecules, and its forecasting accuracy was assessed through statistical analysis. The research underscores the molecular attributes that influence the inhibitory potency of cyanopyrrolidine derivatives, as identified by pharmacophore modeling for interaction with the DPP-IV receptor.
Amorphous solids generally exhibit higher solubility compared to crystalline forms due to their lack of long-range order and greater surface area. Converting a crystalline solid into an amorphous form can enhance its solubility.
When a crystalline substance is heated to its melting point (Tm), it transforms from a solid to a liquid state and can revert upon cooling. Swift cooling below Tm can prevent crystallization, yielding a supercooled liquid. If this supercooled liquid is further cooled to its glass transition temperature (Tg), molecules solidify kinetically, forming a glass. Although molecules in a supercooled liquid have higher mobility compared to those in a glassy state, the latter still maintains some level of mobility.
Because of this inherent mobility, it's advantageous for the glass transition temperature of the active pharmaceutical ingredient to be substantially higher (e.g., at least 20°C, preferably 30°C, or even 40°C) than the actual storage conditions.
Amorphous Saxagliptin, with a relatively low Tg of approximately 54°C, tends to revert to a crystalline state under storage conditions. Elevating the Tg of the amorphous form is essential to prevent such recrystallization. This can be achieved by blending the API with a secondary component, usually polymers that reduce the mobility of Saxagliptin molecules and hinder recrystallization.
The spray drying technique can be employed in two ways to create glass solutions: utilizing Saxagliptin as a salt or as a free base in situ with an acid. This results in Saxagliptin being dispersed within a polymer-formed matrix. Critical Quality Attributes (CQAs) of the drug substance were defined, and strategies to control their impact on product quality were presented. Through risk assessments of the manufacturing process, Critical Process Parameters (CPP) and Key Process Parameters (KPP) were identified. Uni- and multivariate experiments were conducted to define the design space within the studied ranges. Acceptable ranges for all process parameters were established to ensure consistent attainment of defined CQAs. A five-batch campaign within the defined design space at the commercial manufacturing site validated the approach. In essence, the Quality by Design (QbD) approach to saxagliptin drug substance manufacturing yielded enhanced process knowledge and a manufacturing design space that consistently produces high-quality drug substance. The process is considered to be well under control.
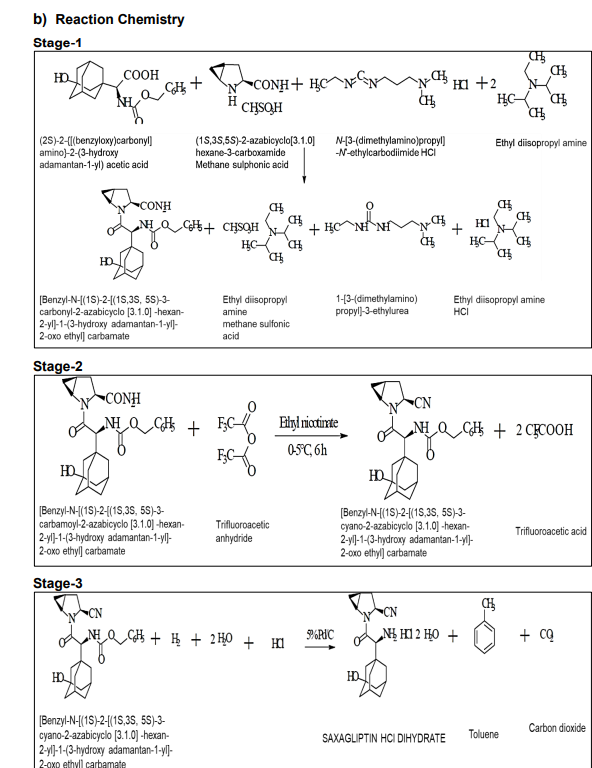
a) Process Description - Stage 1:
The reaction involves (1S, 3S, 5S)-2-(2-azabicyclo-[3.1.0]hexane-3-carboxamide methane sulfonic acid reacting with (2S)-2-{[(benzyloxy)carbonyl]amino}-2-(3-hydroxyadamantan-1-yl)acetic acid in the presence of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 1-hydroxybenzotriazole hydrate, and diisopropylethylamine. This yields Benzyl-N-[(1S)-2-[(1S,3S,5S)-3-carbamoyl-2-azabicyclo [3.1.0]hexan-2-yl]-1-(3-hydroxyadamantan-1-yl)-2-oxoethyl]carbamate (Stage-I).
b) Stage 2:
Benzyl-N-[(1S)-2-[(1S,3S,5S)-3-carbamoyl-2-azabicyclo[3.1.0]hexan-2-yl]-1-(3-hydroxyadamantan-1-yl)-2-oxoethyl]carbamate from Stage-I reacts with trifluoroacetic anhydride in the presence of ethyl nicotinate, leading to the pure Benzyl-N-[(1S)-2-[(1S,3S,5S)-3-cyano-2-azabicyclo[3.1.0]hexan-2-yl]-1-(3-hydroxyadamantan-1-yl)-2-oxoethyl)carbamate (Stage-II).
c) Stage 3:
In this step, Benzyl-N-[(1S)-2-[(1S,3S,5S)-3-cyano-2-azabicyclo[3.1.0]hexan-2-yl]-1-(3-hydroxyadamantan-1-yl)-2-oxoethyl)carbamate (Stage-II) reacts with hydrogen gas in the presence of palladium catalyst and is treated with HCl, resulting in saxagliptin HCl dihydrate tech material.
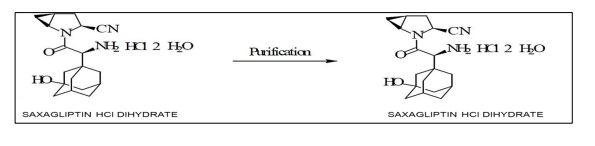
d) Stage 4:
The product from Stage-III (Saxagliptin HCl dihydrate tech) is purified and dried to obtain pure saxagliptin HCl dihydrate product.
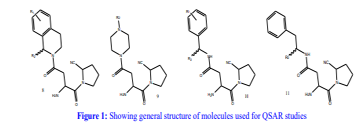
3D QSAR and Pharmacophore Modeling of Substituted Cyanopyrrolidines as Potential Type II Anti-Diabetic Agents
In the realm of medicinal chemistry, 3D QSAR and pharmacophore modeling have been employed to explore the promising potential of substituted cyanopyrrolidines as Type II anti-diabetic agents, particularly as Dipeptidyl Peptidase-IV (DPP-IV) inhibitors. These cyanopyrrolidines, possessing diverse medical functions, have garnered attention for their significant therapeutic applications. Several studies have been conducted to investigate their viability as Type II anti-diabetic agents.
The application of 3D QSAR techniques aimed to unveil the intricate three-dimensional structural elements pivotal for eliciting Type II anti-diabetic activity. The outcomes of the 3D QSAR analysis, characterized by a squared correlation coefficient (r²) of 0.9945 and a cross-validated squared correlation coefficient (q²) of 0.9866, underscore the statistical significance and exceptional predictive capacity of the model. These findings yield critical insights into the structural attributes governing the inhibitory potential of cyanopyrrolidines.
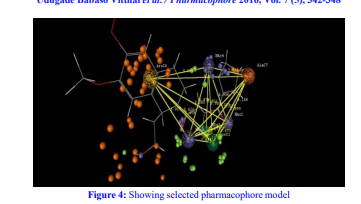
Additionally, pharmacophore modeling was harnessed to discern the essential structural features contributing to the biological efficacy of cyanopyrrolidines. The knowledge garnered from this investigation holds paramount importance for shaping the development of potent Type II anti-diabetic agents, particularly as DPP-IV inhibitors.
Type II diabetes, a prominent metabolic disorder with global prevalence, underscores the significance of this research. This ailment stems from impaired insulin effects on the liver and skeletal muscles, coupled with diminished insulin secretion. Glucagon-like peptide-1 (GLP-1) emerges as an insulinotropic hormone with anti-diabetic potential, marked by glucose-dependent insulin stimulation and glucagon secretion inhibition. However, the rapid inactivation of GLP-1 by Dipeptidyl Peptidase-IV (DPP-IV) curtails its clinical utility. To address this, orally active DPP-IV inhibitors have been pursued to extend GLP-1 activity, resulting in reduced blood glucose levels.
Previous studies involving GLP-1 analogs and DPP-IV inhibitors have shown promise in improving cardiovascular disease outcomes associated with diabetes. However, challenges such as side effects and potency limitations persist. This underscores the potential of computer-aided drug design, as exemplified by quantitative structure-activity relationship (QSAR) studies and pharmacophore modeling. These methodologies shed light on the structural attributes underpinning biological activity.
In the current study, a series of cyanopyrrolidine derivatives were subjected to QSAR studies and pharmacophore modeling using VLife-MDS 4.3 software. The computational analyses were executed on standard hardware and software configurations. The dataset, comprising compounds with reported DPP-IV inhibitory activities, was utilized for model development.
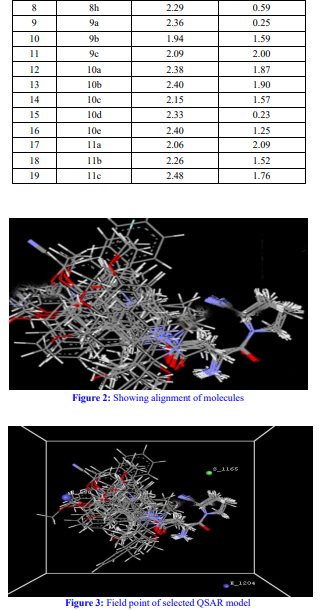
Ligand preparation, molecular alignment, and descriptor generation were integral to the QSAR analysis. A meticulous selection process led to the identification of a robust QSAR model, marked by high correlation coefficients and statistically significant F and p values. Noteworthy descriptors like E-550, S-1165, and E-1204 emerged, revealing steric and electrostatic interactions crucial for anti-diabetic activity. Further, pharmacophore modeling delineated key interaction features between ligands and receptors, offering a blueprint for rational drug design.
In conclusion, this study's integration of 3D QSAR and pharmacophore modeling techniques presents a holistic approach to designing effective Type II anti-diabetic agents. The insights gained from these analyses hold promise for guiding future drug development endeavors, yielding compounds with enhanced potency and improved pharmacological profiles. Ultimately, this work contributes to the pool of knowledge driving the discovery of novel DPP-IV inhibitors with potential therapeutic applications in diabetes management
References
- Metzler, W.J., Yanchunas, J., Weigelt, C., Kish, K., Klei, H.E et al. (2008). Involvement of DPP-IV catalytic residues in enzyme-saxagliptin complex formation. Prot. Sci,17, 240–250.
View at Publisher | View at Google Scholar - Manaithiya, A. Alam, O. Sharma, V. Javed Naim, M. Mittal, S. Khan, I.A. (2021). GPR119 agonists: Novel therapeutic agents for type 2 diabetes mellitus. Bioorg. Chem.113,104998.
View at Publisher | View at Google Scholar - He, L. Wang, J. Ping, F. Yang, N. Huang, et al. (2022). Dipeptidyl peptidase-4 inhibitors and gallbladder or biliary disease in type 2 diabetes: Systematic review and pairwise and network meta-analysis of randomised controlled trials. BMJ, 377, E068882.
View at Publisher | View at Google Scholar - Tomovic, K. Ilic, B.S. Smelcerovic, (2021). A. Structure-activity relationship analysis of cocrystallized gliptin-like pyrrolidine, trifluorophenyl, and pyrimidine-2,4-dione dipeptidyl peptidase-4 inhibitors. J. Med. Chem, 64, 9639–9648.
View at Publisher | View at Google Scholar - Yang, F. Dong, Y. Li, B. Ning, B. Zhao, (2022). Q. Pancreatic safety of DPP-4 inhibitors in type 2 diabetes millitus. Medicine, 101, E29154.
View at Publisher | View at Google Scholar - Wu, D. Jin, F. Lu, W. Zhu, J. Li, et al. (2012). Synthesis, structure-activity relationship, and pharmacophore modeling studies of pyrazole-3-carbohydrazone derivatives as dipeptidyl peptidase IV inhibitors. Chem. Biol. Drug Des, 79:897–906.
View at Publisher | View at Google Scholar - Jones, L. Jones, A.M. (2022). Suspected adverse drug reactions of the type 2 antidiabetic drug class dipeptidyl-peptidase IV inhibitors (dpp4i): Can polypharmacology help explain? Pharmacol. Res. Perspect, 10, E01029.
View at Publisher | View at Google Scholar - Jiang, T. Zhou, Y. Chen, Z. Sun, et al. (2015). Design, synthesis, and pharmacological evaluation of fused β-homophenylalanine derivatives as potent DPP-4 inhibitors. ACS Med. Chem. Lett., 6, 602–606.
View at Publisher | View at Google Scholar - Jeon, W.K. Kang, J. Kim, H.-S. Park, K.W. (2021). Cardiovascular outcomes comparison of dipeptidyl peptidase-4 inhibitors versus sulfonylurea as add-on therapy for type 2 diabetes mellitus: A meta-analysis. J. Lipid Atheroscl., 10, E210.
View at Publisher | View at Google Scholar - Panaro, B.L. Coppage, A.L. Beaudry, J.L. Varin, E.M. Kaur, K. et al. (2019). D.J. Fibroblast activation protein is dispensable for control of glucose homeostasis and body weight in mice. Mol. Metab.19, 65–74.
View at Publisher | View at Google Scholar - Singh, S.K. Manne, N. Pal, (2008). M. Synthesis of (s)-1-(2-chloroacetyl) pyrrolidine-2-carbonitrile: A key intermediate for dipeptidyl peptidase IV inhibitors. Beilstein J. Org. Chem, 4, E20.
View at Publisher | View at Google Scholar - Kridin, K. Avni, O. Damiani, G. Tzur Bitan, D. Onn, et al. (2022). A.D. Dipeptidyl-peptidase IV inhibitor (DPP4I) confers increased odds of bullous pemphigoid even years after drug initiation. Arch. Dermatol. Res., 315, 33–39.
View at Publisher | View at Google Scholar - Yang, S. He, W. Zhao, L. Mi, (2022). Y. Association between use of sodium-glucose cotransporter 2 inhibitors, glucagon-like peptide 1 agonists, and dipeptidyl peptidase 4 inhibitors with kidney outcomes in patients with type 2 diabetes: A systematic review and network meta-analysis. PLoS ONE, 17, E0267025.
View at Publisher | View at Google Scholar - Bösenberg, L.H. van Zyl, (2008). D.G. The mechanism of action of oral antidiabetic drugs: A review of recent literature. J. Endocrinol. Metab. Diab. S. Afr., 13, 80–88.
View at Publisher | View at Google Scholar - Guardado-Mendoza, R. Prioletta, A. Jiménez-Ceja, L.M. Sosale, A. Folli, (2013). F. State of the art paper the role of nateglinide and repaglinide, derivatives of meglitinide, in the treatment of type 2 diabetes mellitus. Arch. Med. Sci. 5: 936–943.
View at Publisher | View at Google Scholar - Rena, G. Hardie, D.G. Pearson, (2017). E.R. The mechanisms of action of metformin. Diabetologia, 60;1577–1585.
View at Publisher | View at Google Scholar - Aryaeian, N. Khorshidi Sedehi, S. Arablou, (2017). T. Polyphenols and their effects on diabetes management: A review. Med. J. Islamic Repub. Iran, 31; 886–892.
View at Publisher | View at Google Scholar - Boath, A.S. Stewart, D. McDougall, (2012). G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem.135, 929–936.
View at Publisher | View at Google Scholar - Firdaus, J.U. Siddiqui, N. Alam, O. Manaithiya, A. Chandra, (2023). K. Pyrazole scaffold-based derivatives: A glimpse of α-glucosidase inhibitory activity, SAR, and route of synthesis. Arch. Pharm, 365P: E2200421.
View at Publisher | View at Google Scholar - Blahova, J. Martiniakova, M. Babikova, M. Kovacova, V. Mondockova, V. et al. (2021). Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals, 14:806.
View at Publisher | View at Google Scholar - 21.Sakr, (2022). H. Review on the significance of quinazolinone derivatives as potent antihyperglycemic agents. Al-Azhar J. Pharm. 65; 50–63.
View at Publisher | View at Google Scholar - Kong, F. Pang, X. Zhao, J. Deng, et al. (2019). X. Hydrolytic metabolism of cyanopyrrolidine DPP-4 inhibitors mediated by dipeptidyl peptidases. Drug Metab. Dispos, 47;238–248.
View at Publisher | View at Google Scholar - Pellegatti, L. Sedelmeier, J. (2015). Synthesis of vildagliptin utilizing continuous flow and batch technologies. Org. Proc. Res. Dev., 19;551–554.
View at Publisher | View at Google Scholar - Omar, B. Ahrén, B. (2014). Pleiotropic mechanisms for the glucose-lowering action of DPP-4 inhibitors. Diabetes, 63;2196–2202.
View at Publisher | View at Google Scholar - Aertgeerts, K. (2004). Crystal structure of human dipeptidyl peptidase IV in complex with a decapeptide reveals details on substrate specificity and tetrahedral intermediate formation. Protein Sci, 13; 412–421.
View at Publisher | View at Google Scholar - Juillerat-Jeanneret, (2014). L. Dipeptidyl peptidase IV and its inhibitors: Therapeutics for type 2 diabetes and what else? J. Med. Chem. 57;2197–2212.
View at Publisher | View at Google Scholar - Al-Abdullah, E.S. Al-Tuwaijri, H.M. Hassan, H.M. Haiba, M.E. Habib, et al. (2014). A.A. Antimicrobial and hypoglycemic activities of novel N-Mannich bases derived from 5-(1-adamantyl)-4-substituted-1,2,4triazoline-3-thiones. Int. J. Mol. Sci. 15; 22995–23010.
View at Publisher | View at Google Scholar - Maladkar, M. Sankar, S. Kamat, (2016). K. Teneligliptin: Heralding change in type 2 diabetes. J. Diabetes Mellitus 6;113–131.
View at Publisher | View at Google Scholar - Nabeno, M. Akahoshi, F. Kishida, H. Miyaguchi, I. Tanaka, Y. Ishii, et al. (2013). T. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem. Biophys. Res. Comm, 434;191–196.
View at Publisher | View at Google Scholar - Kushwaha, R.N. Haq, W.S. Katti, (2014). S.B. Discovery of 17 gliptins in 17-years of research for the treatment of type 2 diabetes: A synthetic overview. Chem. Biol. Interface 4;137–162.
View at Publisher | View at Google Scholar - Deacon, C.F. Mannucci, E. Ahrén, (2012). B. Glycaemic efficacy of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors as add-on therapy to metformin in subjects with type 2 diabetes-A review and meta-analysis. Diabetes Obes. Metab.14;762–767.
View at Publisher | View at Google Scholar - Waget, A. Cabou, C. Masseboeuf, M. Cattan, P. Armanet, M. et al. (2011). Physiological and pharmacological mechanisms through which the DPP-4 inhibitor Sitagliptin regulates glycemia in mice. Endocrinology,152, 3018–3029.
View at Publisher | View at Google Scholar - Vardarli, I. Nauck, M.A. Köthe, L.D. Deacon, C.F. Holst, et al. (2011). A. Foley, J. Inhibition of DPP-4 with vildagliptin improved insulin secretion in response to oral as well as “isoglycemic” intravenous glucose without numerically changing the incretin effect in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 96;945–954.
View at Publisher | View at Google Scholar - Salehi, M. Aulinger, B. Prigeon, R.L. D’Alessio, D.A. Effect of endogenous GLP-1 on insulin secretion in type 2 diabetes. Diabetes, 59;1330–1337.
View at Publisher | View at Google Scholar - Kumar, S. Mittal, A. Mittal, (2021). A. A review upon medicinal perspective and designing rationale of DPP-4 inhibitors. Bioorg. Med. Chem. 46; E116354.
View at Publisher | View at Google Scholar - Idris, I. Donnelly, (2007). R. Dipeptidyl peptidase-IV inhibitors: A major new class of oral antidiabetic drug. Diabetes Obes. Metab. 9;153–165.
View at Publisher | View at Google Scholar - Kushwaha, R.N. Haq, W. Katti, (2014). S.B. Sixteen-years of clinically relevant dipeptidyl peptidase-IV (DPP-IV) inhibitors for treatment of type-2 diabetes: A perspective. Curr. Med.Chem. 21, 4013–4045.
View at Publisher | View at Google Scholar - Ahrén, B. Schmitz, (2004). O. GLP-1 receptor agonists and DPP-4 inhibitors in the treatment of type 2 diabetes. Hormone Metab. Res. 36; 867–876.
View at Publisher | View at Google Scholar - Chaplin, S. Farooqi, (2014). A. Alogliptin-a new DPP-4 inhibitor for type 2 diabetes. Prescriber, 25;15–16.
View at Publisher | View at Google Scholar - Zhu, Y. Meng, X. Cai, Z. Hao, Q. Zhou, (2017). W. Synthesis of phenylpyridine derivatives and their biological evaluation toward dipeptidyl peptidase-4. Chem. Heterocyc. Comp, 53; 350–356.
View at Publisher | View at Google Scholar - Hansen, K.B. Hsiao, Y. Xu, F. Rivera, N. Clausen, A. et al. (2009). Highly efficient asymmetric synthesis of sitagliptin. J. Am. Chem. Soc., 131, 8798–8804.
View at Publisher | View at Google Scholar - Peng, F. Chen, Y. Chen, C.Y. Dormer, P.G. Kassim, et al. (2017). Asymmetric formal synthesis of the long-acting DPP-4 inhibitor omarigliptin. J. Org. Chem.82, 9023–9029.
View at Publisher | View at Google Scholar - Chung, J.Y. Scott, J.P. Anderson, C. Bishop, B. Bremeyer, et al. (2015). Evolution of a manufacturing route to omarigliptin, a long-acting DPP-4 inhibitor for the treatment of type 2 diabetes. Org. Proc. Res. Dev., 19;1760–1768.
View at Publisher | View at Google Scholar - Kumar, N. Devineni, S.R. Aggile, K. Gajjala, P.R. Kumar, (2017). Facile new industrial process for synthesis of teneligliptin through new intermediates and its optimization with control of impurities. Res. Chem. Intermed. 44, 567–584.
View at Publisher | View at Google Scholar - Biftu, T. Sinha-Roy, R. Chen, P. Qian, X. Feng, D. Kuethe, et al. (2014). Omarigliptin (MK-3102): A novel long-acting DPP-4 inhibitor for once-weekly treatment of type 2 diabetes. J. Med. Chem., 57, 3205–3212.
View at Publisher | View at Google Scholar - Castaldi, M. Baratella, M. Menegotto, I.G. Castaldi, G. Giovenzana, (2017). G.B. A concise and efficient synthesis of vildagliptin. Tetrahedron Lett., 58, 3426–3428.
View at Publisher | View at Google Scholar - Zhang, C. Ye, F. Wang, J. He, P. Lei, M. Huang, et al. (2020). Design, synthesis, and evaluation of a series of novel super long-acting DPP-4 inhibitors for the treatment of type 2 diabetes. J. Med. Chem. 63;7108–7126.
View at Publisher | View at Google Scholar - Kim, D. Wang, L. Beconi, M. Eiermann, et al. (2005). (2R)-4-Oxo-4-[3-(trifluoromethyl)-5,6-dihydro [1, 2, 4]triazolo[4,3-a] pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: Molecules 2023, 28, 5860 42 of 43 A potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem., 48, 141–151.
View at Publisher | View at Google Scholar - Wu, W.L. Hao, J. Domalski, M. Burnett, D.A. Pissarnitski, D. Zhao, et al. (2016). Discovery of novel tricyclic heterocycles as potent and selective DPP-4 inhibitors for the treatment of type 2 diabetes. ACS Med. Chem. Lett., 7; 498–501.
View at Publisher | View at Google Scholar - Liang, G.B. Qian, X. Biftu, T. Singh, S. Gao, et al. (2008). Discovery of new binding elements in DPP-4 inhibition and their applications in novel DPP-4 inhibitor design. Bioorg. Med. Chem. Lett.18, 3706–3710.
View at Publisher | View at Google Scholar - Luo, N. Fang, X. Su, M. Zhang, X. Li, D. Li, et al. (2020). Z. Design, synthesis and sar studies of novel and potent dipeptidyl peptidase 4 inhibitors. Chin. J. Chem. 39, 115–120.
View at Publisher | View at Google Scholar - Al-Wahaibi, L.H. Joubert, J. Blacque, O. Al-Shaalan, N.H. El-Emam, (2019). A.A. Crystal structure, Hirshfeld surface analysis and DFT studies of 5-(adamantan-1-yl)-3-[(4-chlorobenzyl) sulfanyl]-4-methyl-4H-1,2,4-triazole, a potential 11β-HSD1 inhibitor. Sci. Rep., 9, E56331.
View at Publisher | View at Google Scholar - Spasov, A.A. Vasil’ev, P.M. Babkov, D.A. Prokhorova, T.Y. Sturova, et al. (2017). M.R. New dipeptidyl peptidase 4 inhibitors among adamantane derivatives. Russ. J. Bioorg. Chem., 43; 449–455.
View at Publisher | View at Google Scholar - 54.Arulmozhiraja, S. Matsuo, N. Ishitsubo, E. Okazaki, S. Shimano, (2016). H. Comparative binding analysis of dipeptidyl peptidase IV (DPP-4) with anti-diabetic drugs -An Ab initio fragment molecular orbital study. PLoS ONE ;11, E0166275.
View at Publisher | View at Google Scholar - Villhauer, E.B. Brinkman, J.A. Naderi, G.B. Burkey, B.F. Dunning, et al. (2003). A potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 46;2774–2789.
View at Publisher | View at Google Scholar - Wilcken, R. Zimmermann, M.O. Lange, A. Joerger, (2013). F.M. Principles and applications of halogen bonding in medicinal chemistry and chemical biology. J. Med. Chem., 56;1363–1388.
View at Publisher | View at Google Scholar - Sever, B. Soybir, H. Görgülü, S. Cantürk, Z. Altıntop, (2020). M.D. Pyrazole incorporated new thiosemicarbazones: Design, synthesis and investigation of DPP-4 inhibitory effects. Molecules; 25;5003.
View at Publisher | View at Google Scholar - Narsimha, S. Battula, K.S. Ravinder, M. Reddy, Y.N. Nagavelli, V.R. Design, (2020). synthesis and biological evaluation of novel 1,2,3-triazole-based xanthine derivatives as DPP-4 inhibitor. J. Chem. Sci., 132, E59.
View at Publisher | View at Google Scholar - Brigance, R.P. Meng, W. Fura, A. Harrity, T. Wang, A. Zahler, et al. (2010). L.G. Synthesis and SAR of azolopyrimidines as potent and selective dipeptidyl peptidase-4 (DPP4) inhibitors for type 2 diabetes. Bioorg. Med. Chem. Lett. 20; 4395–4398.
View at Publisher | View at Google Scholar - Mourad, A.A.E. Khodir, A.E. Saber, S. Mourad, (2021). M.A.E. Novel potent and selective DPP-4 inhibitors: Design, synthesis and molecular docking study of dihydropyrimidine phthalimide hybrids. Pharmaceuticals,14;144.
View at Publisher | View at Google Scholar - Fang, Y. Zhang, S. Wu, W. Liu, Y. Yang, et al. (2020). Design and synthesis of tetrahydropyridopyrimidine derivatives as dual GPR119 and DPP-4 modulators. Bioorg. Chem. 94, E103390.
View at Publisher | View at Google Scholar - Deng, X. Han, L. Zhou, J. Zhang, H. Li, (2017). Q. Discovery of triazole-based uracil derivatives bearing amide moieties as novel dipeptidyl peptidase-IV inhibitors. Bioorg. Chem. 75, 357–367.
View at Publisher | View at Google Scholar - Li, Q. Deng, X. Jiang, N. Meng, L. Xing, J. Jiang, (2021). Y. Identification and structure–activity relationship exploration of uracil-based benzoic acid and ester derivatives as novel dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Eur. J. Med. Chem. 225, E113765.
View at Publisher | View at Google Scholar - Syam, Y.M. Anwar, M.M. Abd El-Karim, S.S. Elseginy, S.A. Essa, (2021). New quinoxaline compounds as DPP-4 inhibitors and hypoglycemics: Design, synthesis, computational and bio-distribution studies. RSC Adv. 11, 36989–37010.
View at Publisher | View at Google Scholar - Fuh, M.T. Tseng, C.C. Li, S.M. Tsai, S.E. Chuang, Wong, et al. (2021). F. Design, synthesis and biological evaluation of glycolamide, glycinamide, and β-amino carbonyl 1,2,4-triazole derivatives as DPP-4 inhibitors. Bioorg. Chem., 114, E105049.
View at Publisher | View at Google Scholar - Schwehm, C. Li, J. Song, H. Hu, X. Kellam, B. Stocks, (2015). M.J. Synthesis of new DPP-4 inhibitors based on a novel tricyclic scaffold. ACS Med. Chem. Lett. 6, 324–328.
View at Publisher | View at Google Scholar - Shu, C. Ge, H. Song, M. Chen, J.-H. Zhou, et al. (2014). Discovery of imigliptin, a novel selective DPP-4 inhibitor for the treatment of type 2 diabetes. ACS Med. Chem. Lett., 5, 921–926.
View at Publisher | View at Google Scholar - Chen, X.W. He, Z.X. Zhou, Z.W. Yang, T. Zhang, et al. (2015). F.S. Clinical pharmacology of dipeptidyl peptidase 4 inhibitors indiacted for the treatment of type 2 diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 42, 999–1024.
View at Publisher | View at Google Scholar - 69.Borghetti, G. Lewinski, D.V. Eaton, D.M. Sourji, H. Houser, et al. (2018). M. Diabetic cardiomyopathy: Current and Future Therapies. Beyond Glycemic Control. Front. Physiol. 9, 1514.
View at Publisher | View at Google Scholar - Saini, K. Sharma, S. Khan, (2023). K. DPP-4 inhibitors for treating T2DM-hype or hope? an analysis based on the current literature. Front. Mol. Biosci., 10, 625.
View at Publisher | View at Google Scholar - Capuano, A. Sportiello, L. Maiorino, M.I. Rossi, F. Giugliano, et al. (2013). K. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapy-focus on alogliptin. Drug Des. Dev. Ther. 7, 989–1001.
View at Publisher | View at Google Scholar - Watanabe, Y.S. Yasuda, Y. Kojima, Y. Okada, S. Motoyama, et al. (2015). a potent dipeptidyl peptidase IV inhibitor: Its single-crystal structure and enzyme interactions. J. Enzym. Inhib. Med. Chem.30, 981–988.
View at Publisher | View at Google Scholar - Sharma, M. Gupta, M. Singh, D. Kumar, M. Kaur, P. Synthesis, (2013). evaluation and molecular docking of prolyl-fluoropyrrolidine derivatives as dipeptidyl peptidase IV inhibitors. Chem. Biol. Drug Des.82;156–166.
View at Publisher | View at Google Scholar - Wallace, M.B. Feng, J. Zhang, Z. Skene, R.J. Shi, et al. (2008). S.L. Structure-based design and synthesis of benzimidazole derivatives as dipeptidyl peptidase IV inhibitors. Bioorg. Med. Chem. Lett.18, 2362–2367.
View at Publisher | View at Google Scholar - Patel, B.D. Ghate, M.D. (2014). Recent approaches to medicinal chemistry and therapeutic potential of dipeptidyl peptidase-4 (DPP-4) inhibitors. Eur. J. Med. Chem. 74;574–605.
View at Publisher | View at Google Scholar - Lotfy, M. Singh, J. Kalász, H. Tekes, K. Adeghate, (2011). E. Medicinal chemistry and applications of Incretins and DPP-4 inhibitors in the treatment of type 2 diabetes mellitus. Open Med. Chem. J. 5; 82–92.
View at Publisher | View at Google Scholar - Syam, Y.M. El-Karim, S.S. Nasr, T. Elseginy, S.A. Anwar, et al. (2019). Design, synthesis and biological evaluation of Spiro cyclohexane-1,2- quinazoline derivatives as potent dipeptidyl peptidase IV inhibitors. Mini-Rev. Med. Chem.19, 250–269.
View at Publisher | View at Google Scholar - Li, N. Wang, L.J. Jiang, B. Li, X.-Q. Guo, et al.(2018). D.Y. Recent progress of the development of dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes mellitus. Eur. J. Med. Chem.,151;145–157.
View at Publisher | View at Google Scholar - Li, Q. Zhou, M. Han, L. Cao, Q. Wang, X. Zhao, et al. (2015). H. Design, synthesis and biological evaluation of imidazo[1, 2-a]pyridine derivatives as novel DPP-4 inhibitors. Chem. Biol. Drug Des. 86;849–856.
View at Publisher | View at Google Scholar - Li, N. Wang, L.J. Jiang, B. Guo, S.J. Li, X.Q. Chen, D.Y. Design, et al. (2018). synthesis and biological evaluation of n0ovel pyrimidinedione derivatives as DPP-4 inhibitors. Bioorg. Med. Chem. Lett. 28;2131–2135.
View at Publisher | View at Google Scholar - Makrilakis, K. (2019). The role of DPP-4 inhibitors in the treatment algorithm of type 2 diabetes mellitus: When to select, what to expect. Int. J. Environ. Res. Public Health,16;2720.
View at Publisher | View at Google Scholar - Savage, S.A. Jones, G.S. Kolotuchin, S. Ramrattan, S.A. Vu, (2009). R.E. Preparation of Saxagliptin, a Novel DPP-IV Inhibitor. Org. Proc. Res. Dev. 13;1169–1176. [CrossRef]
View at Publisher | View at Google Scholar

 Clinic
Clinic
