Review Article | DOI: https://doi.org/10.31579/2835-835X/059
Exploring the Antimicrobial Effects of Pippali (Piper longum) and Haritaki (Terminalia chebula) Against Human Microflora
- Pathy Saihithi Sarma *
- Rachna Chaturvedi
- Jyoti Prakash
Amity Institute of Biotechnology, Amity University, Uttar Pradesh (AUUP), Lucknow Campus Malhaur Railway Station Road, Gomti Nagar, Lucknow, Uttar Pradesh.
*Corresponding Author: Pathy Saihithi Sarma, Amity Institute of Biotechnology, Amity University, Uttar Pradesh (AUUP), Lucknow Campus Malhaur Railway Station Road, Gomti Nagar, Lucknow, Uttar Pradesh.
Citation: Pathy S. Sarma, Rachna Chaturvedi, Jyoti Prakash, (2024), Exploring the Antimicrobial Effects of Pippali (Piper longum) and Haritaki (Terminalia chebula) Against Human Microflora, Clinical Trials and Case Studies, 3(3); DOI:10.31579/2835-835X/059.
Copyright: © 2024, Pathy Saihithi Sarma. This is an open-access artic le distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: 17 February 2024 | Accepted: 15 April 2024 | Published: 23 May 2024
Keywords: antimicrobial; human microflora; agar disc diffusion assays; minimum inhibitory concentration; synergistic effects; additive effects; phytochemical composition; bioactive compounds
Abstract
This study investigates the potential antimicrobial properties of Pippali (Piper longum) and Haritaki (Terminalia chebula) against human microflora. Microbial infections pose a significant threat to human health, and the search for alternative antimicrobial agents has gained prominence. Pippali and Haritaki, two traditional medicinal plants in Ayurveda, have been historically recognized for their diverse pharmacological properties. The aim of this research is to assess the antimicrobial efficacy of Pippali and Haritaki extracts against a range of human-associated microorganisms.The study employs various microbiological techniques, including agar disc diffusion assays,minimum inhibitory concentration (MIC) and Minimum lethal concentration(MLC) determination, to evaluate the inhibitory effects of Pippali and Haritaki extracts. Additionally, the potential synergistic or additive effects of combining these herbal extracts with conventional antimicrobial agents are explored. The investigation also delves into the phytochemical composition of Pippali and Haritaki extracts, aiming to identify specific bioactive compounds responsible for their antimicrobial activity. Preliminary findings suggest that Pippali and Haritaki extracts exhibit notable antimicrobial activity against a spectrum of human microflora, including both Gram-positive and Gram-negative bacteria. The results of this study contribute valuable insights into the therapeutic potential of these traditional medicinal plants as natural antimicrobial agents, paving the way for further research and potential development of alternative strategies to combat microbial infections
Introduction
Otitis externa, a prevalent ailment affecting the external auditory canal and auricle, has become a noteworthy global health concern, impacting 5% to 20% of patients seeking care at ear, nose, and throat (ENT) clinics3. The rise of drug-resistant strains, exacerbated by the indiscriminate use of commercial antimicrobial drugs, has spurred a worldwide exploration for alternative sources of antimicrobials. In this pursuit, India, celebrated for its profound traditional herbal knowledge, emerges as a promising repository. Among the diverse array of potential candidates, Terminalia chebula[15], commonly known as Black Myrobalan, stands out for its extensively documented medicinal uses, making it a focal point in the quest for substitutes to synthetic agents.The investigation into plant-derived compounds has played a pivotal role in reshaping drug discovery paradigms, especially in addressing challenges posed by bacterial diversity and antibiotic resistance. This is particularly relevant for strains such as Pseudomonas, where conventional antibiotics are witnessing a decline in effectiveness [16-17]. Medicinal plants, typified by Piper longum, have demonstrated efficacy against bacterial diseases, presenting a potential avenue for the development of potent antibacterial agents. The chemical analysis of Piper longum has unveiled constituents like piperine, contributing to both bioavailability and therapeutic diversity4.This study endeavors to contribute meaningfully to the ongoing search for novel antibacterial agents [18-19] by evaluating the antimicrobial potential of T. chebula fruits and isolating key constituents from Piper longum fruits. Aligned with the historical use of plant-derived compounds in traditional medicine5, these efforts hold promise for the development of safer and more effective therapeutic agents against microbial infections. Moreover, the unique cultivation conditions of Piper longum, thriving in limestone soil in the Cherrapunji region with heavy rains and high humidity, add an intriguing dimension to this exploration. The detailed cultivation practices and harvesting methods underscore the significance of environmental factors [20] in obtaining potent medicinal compounds from plants like Piper longum.As a native plant in India, Terminalia chebula, commonly known as Karakkaya in Telugu (Harad in Hindi), has beentraditionally employed for its medicinal properties20, particularly as a cough reliever. The study also emphasizes the extensive use of Terminalia chebula in Ayurvedic formulations for infectious diseases, chronic ulcers, fungal infections of the skin, and its role in promoting longevity, immunity, and overall body resistance against diseases. The "king of medicines"6 holds immense potential for contemporary medicine in combating microbial infections and contributing to global health [21-22]. Haritaki (Terminalia chebula) and Pippali (Piper longum) are traditional herbs in Ayurveda known for their potential benefits in promoting oral health. These herbs are believed to possess anti-inflammatory properties, contributing to the maintenance of healthy gums [23]. They can be incorporated into various oral care formulations to support gingival well-being. Haritaki is traditionally recognized for its analgesic properties and is occasionally applied topically to alleviate toothaches. The combination of Haritaki and Pippali may be integrated into natural toothpaste or mouthwash formulations [24]. Haritaki's astringent properties, along with Pippali's believed antimicrobial effects, can collectively contribute to oral hygiene by inhibiting the growth of harmful microorganisms [8] in the mouth.Moreover, the antimicrobial characteristics 7of Pippali and the astringent nature of Haritaki may help address issues related to bad breath. Overall, the synergistic properties of these herbs make them potential candidates for holistic oral care [8-9] .Pippali fruit, a staple in traditional medicine, is utilized for various ailments such as cough, bronchitis, asthma, respiratory infections, constipation, gonorrhea, diarrhea, cholera, malaria, hepatitis, stomach-ache, spleen diseases, and tumors. Its primary strength lies in treating respiratory conditions like colds, coughs, and bronchitis, acting as a counter-irritant to reduce inflammation. Particularly effective against asthma, it not only fights infections but also thins phlegm and alleviates congestion, reducing the intensity and frequency of asthma attacks [10].
Process of preparing plant extractions:
Plant Collection: The fruits of Piper longum was obtained from the local markets of Madugula and Terminalia chebula from the local markets of Visakhapatnam.
Processing and Extraction of Pippali and Haritaki Fruits:
a) We washed the Pippali and Haritaki fruits thoroughly under running tap water and then rinsed them with sterile distilled water. After that, we dried them completely in a hot air oven at 50°C. Once dried, we ground them into a fine powder using a sterilized mixer grinder and stored the powder in sealed containers.
b) Next, we took 0.98 grams of the dried fruit powder from both Pippali and Haritaki and mixed it with 9.8 milliliters of ethanol in a conical flask. We sealed the flask with cotton wool and placed it on a rotary shaker set at 120 rotations per minute for 5 days to make sure all the active compounds were fully extracted.
c) Similarly, we prepared n-hexane extracts of Pippali and Haritaki using the same method.
d) After extraction, we filtered the extracts using Whatman filter paper. Then, we further purified the filtrate by centrifuging it at 4000 times the force of gravity for 5 minutes.
e) Finally, we stored the crude extracts in sealed containers at 4°C to preserve their potency.
Preparation of Sterile Discs:
The Sterile filter paper discs were prepared from Whatman’s No.1 filter paper. Discs of 6mm size were prepared in Petri plate and sterilized in an autoclave at 121°C for 15 minutes. Paper discs were soaked and allowed to stand for one hour to ensure complete saturation and air dried.
Antimicrobial activity
Bacterial strains: The fruit extracts were dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mg/mL and evaluated for antibacterial activity using the agar disc diffusion assay. The bacterial culture in Muller Hinton broth was adjusted to a final inoculum density of 1x107colony forming units per milliliter (CFU/mL) using the 0.5 McFarland standard, and plated on molten Muller Hinton agar (MHA) plates. Streptomycin 25served as the positive control in this assay. After 24 hours of incubation at 37 °C, the antibacterial activity was determined by measuring the diameter of inhibition zones around each disc containing either the plant extracts or the antibiotic. Each test was performed in triplicate to ensure the reliability of the results.
Determination of minimum inhibitory concentration (MIC):
The MIC of the fruit extracts against the tested bacteria was determined by broth micro-dilution procedure to find the lowest concentration of the extract at which no growth was visible. Stock solutions (10.24 mg/mL) of above extracts (Pippali and Haritaki) were prepared in DMSO, and serially diluted in Muller Hinton broth at concentrations of 5.12, 2.56, 1.28, 0.64, 0.32, 0.16, 0.08 and 0.04 mg/mL in a 96-well microtitre plate. The broth culture containing 0.5 McFarland (1x108 CFU/mL) inoculum density was then introduced to each of the microtitre wells at 1:10 ratio to maintain final inoculum density of 1x107 CFU/mL. Microtitre plates were incubated for 18 h at 37 °C, and the presence of visible growth in each well was inferred by measuring OD at 630 nm using ELISA reader.
Determination of the Minimum Lethal Concentration (MLC):
The MLC (Minimum Lethal Concentration) for the antimicrobials was determined using the dilution in broth method. After 48 hours of incubation at 37°C, 0.1 mL of the solution was extracted from wells in the microtiter plates (Thermo Scientific) where no growth was observed. This sample was then plated onto Trypticase soy agar plates and further incubated for 48 hours at 37°C. The MLC was defined as the lowest concentration of the antimicrobial at which no colonies were observed on the agar plates.Given that the detection limit of this method is 10 cfu/mL, the absence of growth on the Trypticase soy agar plates indicated that the concentration of bacteria was below this limit. Starting from an initial concentration of 105 cfu/mL, the MLC effectively reduced the bacterial count to below 10 cfu/mL. Therefore, the MLC represented the minimum concentration of the antimicrobial required to deactivate more than 99.99% of the bacteria present.Each strain and antimicrobial compound were tested in triplicate to ensure consistency and reliability of the results.
Discussion
The study uses extracts from Terminalia chebula and Piper longum fruits, employing various solvents like n-Hexane and ethanol along with antibiotic like streptomycin. The results indicate significant antibacterial properties against various bacterial strains [11-12]. A comparison study on Terminalia chebula fruits revealed antibacterial activity against tested strains, with the ethanolic extract being the most effective. The variations in results can be attributed to different plant materials, solvents, antibiotics, and bacterial strains used in each study13.
Bacteria |
Ethanolic Extract |
n- Hexane Extract |
Standard (Streptomycin) |
Staphylococcus epidermidis |
17 |
10 |
25 |
Streptococcus mutans |
10 |
12 |
18 |
Staphylococcus aureus |
15 |
11 |
20 |
Escherichia coli |
23 |
21 |
27 |
Streptococcus pneumoniae |
21 |
20 |
21 |
Micrococcus luteus |
13 |
10 |
26 |
Pseudomonas aeruginosa |
12 |
11 |
18 |
Propionibacterium acnes |
11 |
09 |
22 |
Study 1: Utilizes various solvents like n-Hexane, ethanol, and antibiotic like streptomycin.
Diameter of Zone of Inhibition (mm)
Antibacterial properties of extracts of Piper longum fruits
Diameter of Zone of Inhibition (mm)
Bacteria |
Ethanolic Extract |
n- Hexane Extract |
Standard (Streptomycin) |
Staphylococcus epidermidis |
21 |
20 |
22 |
Streptococcus mutans |
17 |
18 |
17 |
Staphylococcus aureus |
22 |
15 |
25 |
Escherichia coli
|
15 |
17 |
24 |
Streptococcus pneumoniae |
18 |
17 |
20 |
Micrococcus luteus |
16 |
14 |
18 |
Pseudomonas aeruginosa |
17 |
16 |
19 |
Propionibacterium acnes |
24 |
19 |
28 |
Antibacterial properties of extracts of Terminalia chebula fruit
Bacteria |
mg/ml |
Ethanolic extract |
n- hexane extract |
Staphylococcus epidermidis |
MIC MLC |
3.12 6.25 |
4.25 6.12 |
Streptococcus mutans |
MIC MLC |
12.5 20.5 |
10.8 22.2 |
Staphylococcus aureus |
MIC MLC |
4.25 15.5 |
6.20 13.33 |
Escherichia coli
|
MIC MLC |
25.5 30.8 |
28.6 32.6 |
Streptococcus pneumoniae |
MIC MLC |
20.5 45.5 |
23.2 55.6 |
Micrococcus luteus |
MIC MLC |
8.54 48.42 |
12.25 50.00 |
Pseudomonas aeruginosa
|
MIC MLC |
39.46 58.60 |
57.50 65.00 |
Propionibacterium acnes
|
MIC MLC |
0.78 1.56 |
0.98 2.56 |
Antibacterial properties of extracts of Terminalia chebula fruits
Bacteria |
mg/ml |
Ethanolic extract |
n- hexane extract |
Staphylococcus epidermidis |
MIC MLC |
3.12 6.25 |
4.25 6.12 |
Streptococcus mutans |
MIC MLC |
12.5 20.5 |
10.8 22.2 |
Staphylococcus aureus |
MIC MLC |
4.25 15.5 |
6.20 13.33 |
Escherichia coli
|
MIC MLC |
25.5 30.8 |
28.6 32.6 |
Streptococcus pneumoniae |
MIC MLC |
20.5 45.5 |
23.2 55.6 |
Micrococcus luteus |
MIC MLC |
8.54 48.42 |
12.25 50.00 |
Pseudomonas aeruginosa
|
MIC MLC |
39.46 58.60 |
57.50 65.00 |
Propionibacterium acnes
|
MIC MLC |
0.78 1.56 |
0.98 2.56 |
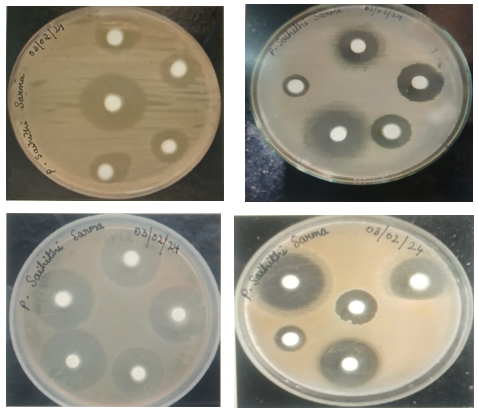
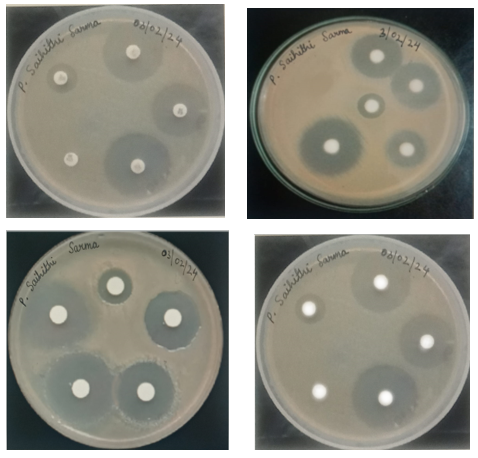
Figure: Zone of Inhibition of different microorganisms
Antibacterial properties of extracts of Pippali fruits:
The ethanolic extract of Pippali exhibited a larger zone of inhibition compared to the n-hexane extract. Specifically, the zone of inhibition for the ethanolic extract against E. coli was 23 mm, while the smallest inhibition zone was observed against Streptococcus mutans, measuring 10 mm.On the other hand, the n-hexane extract of Pippali displayed a greater zone of inhibition against E. coli, measuring 21 mm. The smallest inhibition zone for the n-hexane extract was observed against Propionibacterium acnes, measuring 09 mm. Streptomycin acts as a control in this experiment.
Antibacterial properties of extracts of Haritaki fruits:
The ethanolic extract of Haritaki exhibited a significant zone of inhibition against Propionibacterium acnes, measuring 24 mm, while displaying the smallest inhibition zone against E. coli at 15 mm.Similarly, the n-hexane extracts of Haritaki demonstrated notable inhibitory activity against Staphylococcus epidermidis, with a zone of inhibition measuring 20 mm, while exhibiting the lowest inhibition zone against Micrococcus luteus at 14 mm. The n-hexane extracts of Haritaki exhibited a larger inhibition zone of 18mm against Streptococcus mutans compared to the ethanolic extracts of Haritaki. This trend was also observed in the case of E.coli. Streptomycin acts as a control in this experiment.
Conclusion
In conclusion, the studies on isolated constituents from Piper longum and Terminalia chebula fruits offer valuable insights into the antibacterial properties of these fruit extracts. The findings suggest that both Pippali and Haritaki extracts possess antimicrobial properties against the tested bacterial strains. The choice of solvent (ethanol vs. n-hexane) influences the potency of the extracts against specific bacterial species. Additionally, Haritaki extracts tend to exhibit stronger inhibition against certain bacteria compared to Pippali extracts. Further research could explore the mechanisms underlying these differences and their potential applications in antimicrobial therapies.
References
- Aneja KR, Joshi R (2009). Evaluation of antimicrobial properties of fruit extracts of Terminalia chebula against dental caries pathogens. Jundishapur J Microbiol 2: 105-11.
View at Publisher | View at Google Scholar - Khokra SL, Prakash O, Jain S, Aneja KR, Dhingra Y (2008). Essential oil composition and antibacterial studies of Vitex negundo Linn.extracts. Ind J Phar Sci. 70: 522–6.
View at Publisher | View at Google Scholar - Aneja KR, Joshi R, Sharma C (2009). Antimicrobial activity of Dalchini (Cinnamomum zeylanicum bark) extracts on some dental caries pathogens. J Pharm Res. 1387-90.
View at Publisher | View at Google Scholar - Aneja KR, Joshi R, Sharma C (2010). In vitro antimicrobial activity of Sapindus mukorossi and Emblica officinalis against dental caries pathogens. Ethnobot Leaflts.14: 402-12.
View at Publisher | View at Google Scholar - Saleem M, Kim HJ, Ali MS, Lee YS (2005). An update on bioactive plant lignans. Nat Prod Rep 22: 696-716.
View at Publisher | View at Google Scholar - Thongson C, Davidson PM, Mahakarrchanakul W, Weiss J (2004). Antimicrobial activity of ultrasound-assisted solvent-extracted spices. Lett Appl Microbiol. 39: 401-6.
View at Publisher | View at Google Scholar - Parmar VS, Jain SC, Bisht KS, Jain R, Taneja P, Jha A et al (1997). Phytochemistry of the genus Piper. Phytochem. 46: 597-673.
View at Publisher | View at Google Scholar - Usia T, Watabe T, Kadota S, TezukaY (2005). Potent CYP3A4 inhibitory constituents of Piper cubeba. J Nat Prod. 68: 64-8.
View at Publisher | View at Google Scholar - Bastos JK, Carvalho JCT, de Souza GHB, Pedrazzi AHP, Sarti SJ (2001). Antiinfla mmatory activity of cubebin, a lignan from the leaves of Zanthoxyllum naranjillo Griseb. J Ethnopharmacol 75: 279-82.
View at Publisher | View at Google Scholar - De Souza VA, Da Silva R, Pereira AC, Royo VD, Saraiva J, Montanheiro M et al(2005). Trypanocidal activity of (-)-cubebin derivatives against free amastigote forms of Trypanosoma cruzi. Bioorgan Med Chem Lett.; 15: 303-7.
View at Publisher | View at Google Scholar - Atal CK, Zutshi U, Rao PG (1981). Scientific evidence on the role of Ayurvedic herbals on bioavailability of drugs. J Ethnopharmacol. 4: 229-32.
View at Publisher | View at Google Scholar - Sunila ES, Kuttan G (2004). I mmunomodulatory and antitumor activity of Piper longum Linn. and piperine. J Ethnopharmacol. 90: 339-46.
View at Publisher | View at Google Scholar - Mishra P (2010). Isolation, spectroscopic characterization and computational modelling of chemical constituents of Piper longum natural product. Int J Pharma Sci Rev Res. 2: 78-86.
View at Publisher | View at Google Scholar - V. Subhose, P. Srinivas, and A. Narayan (2005), “Basic principles of pharmaceutical science in Ayurveda,” ˇ Bulletin of the Indian Institute of History of Medicine, vol. 35, no. 2, pp. 83–92.
View at Publisher | View at Google Scholar - B. Ballabh and O. P. Chaurasia, “Traditional medicinal plants of cold desert Ladakh-Used in treatment of cold, cough and fever,” Journal of Ethnopharmacology, vol. 112, no. 2, pp. 341–345, 2007.
View at Publisher | View at Google Scholar - M. M. Pandey, S. Rastogi, and A. K. S. Rawat (2008), “Indian herbal drug for general healthcare: an overview,” The Internet Journal of Alternative Medicine.
View at Publisher | View at Google Scholar - B. Patwardhan, D. Warude, P. Pushpangadan, and N. Bhatt (2005). “Ayurveda and traditional Chinese medicine: a comparative overview,” Evidence-Based Complementary and Alternative Medicine, vol. 2, no. 4, pp. 465–473.
View at Publisher | View at Google Scholar - R. P. Samy, S. Ignacimuthu, and A. Sen (1998), “Screening of 34 Indian medicinal plants for antibacterial properties,” Journal of Ethnopharmacology, vol. 62, no. 2, pp. 173–181.
View at Publisher | View at Google Scholar - R. P. Samy and S (2000). Ignacimuthu, “Antibacterial activity of some folklore medicinal plants used by tribals in Western Ghats of India,” Journal of Ethnopharmacology, vol. 69, no. 1, pp. 63–71.
View at Publisher | View at Google Scholar - V. P. Kamboj (2000), “Herbal medicine,” Current Science, vol. 78, no. 1, pp. 35–39.
View at Publisher | View at Google Scholar - T. Rabe and J. Van Staden (1997), “Antibacterial activity of South African plants used for medicinal purposes,” Journal of Ethnopharmacology, vol. 56, no. 1, pp. 81–87.
View at Publisher | View at Google Scholar - D. John (1984), “One hundred useful raw drugs of the Kani tribes of Trivandrum forest division, Kerala, India,” International Journal of Crude Drug Research, vol. 22, no. 1, pp. 17–39.
View at Publisher | View at Google Scholar - J. H. Veale, K. I. Furman, and D. W. Oliver (1992), “South African traditional herbal medicines used during pregnancy and childbirth,” Journal of Ethnopharmacology, vol. 36, no. 3, pp. 185–191.
View at Publisher | View at Google Scholar - C. Anesini and C. Perez (1993), “Screening of plants used in Argentine folk medicine for antimicrobial activity,” Journal of Ethnopharmacology, vol. 39, no. 2, pp. 119–128.
View at Publisher | View at Google Scholar - P. A. Cox (1990), Ciba Foundation Symposium 154, John Wiley & Sons, Chichester, UK.
View at Publisher | View at Google Scholar - P. A. Cox and M. J. Balick (1994), “The ethnobotanical approach to drug discovery,” Scientific American, vol. 270, no. 6, pp. 82–87.
View at Publisher | View at Google Scholar -
View at Publisher | View at Google Scholar

 Clinic
Clinic
